- Norfloxacin
-
Norfloxacin 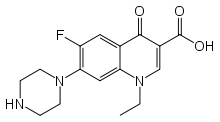
Systematic (IUPAC) name 1-ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1H-quinoline-
3-carboxylic acidClinical data Trade names Noroxin AHFS/Drugs.com monograph MedlinePlus a687006 Pregnancy cat. C(US) Legal status ℞-only (US) Routes Oral Pharmacokinetic data Bioavailability 30 to 40% Protein binding 10 to 15% Metabolism Hepatic Half-life 3 to 4 hours Excretion Renal and fecal Identifiers CAS number 70458-96-7 
ATC code J01MA06 S01AX12 PubChem CID 4539 DrugBank APRD00469 ChemSpider 4380 
UNII N0F8P22L1P 
KEGG D00210 
ChEBI CHEBI:100246 
ChEMBL CHEMBL9 
Chemical data Formula C16H18FN3O3 Mol. mass 319.331 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Norfloxacin is a synthetic chemotherapeutic antibacterial agent[1][2] occasionally used to treat common as well as complicated urinary tract infections.[3] It is sold under various brand names with the most common being Noroxin. In form of ophthalmic solutions it is known as Chibroxin. Norfloxacin is a first generation synthetic fluoroquinolone (quinolone) developed by Kyorin Seiyaku K.K. (Kyorin).[4]
The licensed uses for norfloxacin are quite limited as norfloxacin is to be considered a drug of last resort when all other antibiotics have failed. There are currently only three approved uses in the adult population[5] (one of which is restricted[6]) and the other ineffective due to bacterial resistance. Chibroxin[7] (ophthalmic) is approved for use in children older than one year of age.
Norfloxacin interacts with a number of other drugs, as well as a number of herbal and natural supplements. Such interactions increase the risk of anticoagulation and the formation of non-absorbable complexes, as well as increasing the risk of toxicity.[8]
Norfloxacin is associated with a number of serious and life threatening adverse reactions as well as spontaneous tendon ruptures and irreversible peripheral neuropathy. Such reactions may manifest long after therapy had been completed and in severe cases may result in lifelong disabilities. Hepatoxicity resulting in fatalities has also been reported with the use of norfloxacin.
Contents
History
Since its establishment in 1946, the Japanese Society of Chemotherapy (JSC) has been and currently is involved in the development of synthetic antibacterial agents from nalidixic acid and pipemidic acid, leading to new quinolones.[9] Subsequent work led to the birth of a new era with the introduction of norfloxacin as the first new quinolone in Japan in 1984 and then in many other countries throughout the world. Since the discovery of norfloxacin (1980), around 10,000 new analogues have been described.[10]
Norfloxacin was first patented in 1979.[11] Kyorin granted Merck & Company, Inc., an exclusive license (in certain countries, including the United States), to import and distribute Norfloxacin under the brand name Noroxin. The U.S. Food and Drug Administration (FDA) approved Noroxin for distribution in the United States on October 31, 1986. Since the approval of Noroxin in 1986, there have been numerous upgrades to the warning sections of the package inserts, as well as recent restrictions placed upon the use of Noroxin to treat urinary tract infections (UTIs).[6]
Licensed uses
In the adult population Oral and I.V. Norfloxacin is limited to the treatment of proven bacterial infections. The initial approval by the U.S. Food and Drug Administration (FDA) in 1986 encompassed the following indications:
- Uncomplicated urinary tract infections (including cystitis)
- Complicated urinary tract infections (restricted use) [6]
- Uncomplicated urethral and cervical gonorrhea (however this indication is no longer considered to be effective by some experts due to bacterial resistance) [12][13]
- Prostatitis due to Escherichia coli.
- Syphilis treatment: Norfloxacin has not been shown to be effective in the treatment of syphilis. Antimicrobial agents used in high doses for short periods of time to treat gonorrhea may mask or delay the symptoms of incubating syphilis.[14]
Though the fluoroquinolones are sometimes used to treat typhoid and paratyphoid fever, it should be noted here that norfloxacin had more clinical failures than the other fluoroquinolones (417 participants, 5 trials).[15]
In ophthalmology, Norfloxacin licensed use is limited to the treatment of conjunctival infections caused by susceptible bacteria.[7]
Norfloxacin has been restricted in the Republic of Ireland due to the risks of C. difficile super infections and permanent nerve as well as tendon injuries. It licensed use in acute and chronic complicated kidney infections has been withdrawn as a result.[16]
The European Medicines Agency, also in 2008, had recommended restricting the use of oral norfloxacin to treat urinary infections. CHMP had concluded that the marketing authorizations for norfloxacin, when used in the treatment of acute or chronic complicated pyelonephritis, should be withdrawn because the benefits do not outweigh their risks in this indication. CHMP stated that doctors should not prescribe oral norfloxacin for complicated pyelonephritis and should consider switching patients already taking oral norfloxacin for this type of infection to an alternative antibiotic.[6]
Note: Norfloxacin may be licensed for other uses, or restricted, by the various regulatory agencies worldwide.
Availability
Norfloxacin is available as:
- tablets 400-mg
- eye drops
In most countries, all formulations require a prescription.
See the latest package insert for norfloxacin (Noroxin) for additional details. [5]
Mode of action
Norfloxacin is a broad-spectrum antibiotic that is active against both Gram-positive and Gram-negative bacteria. It functions by inhibiting DNA gyrase, a type II topoisomerase, and topoisomerase IV,[17] enzymes necessary to separate bacterial DNA, thereby inhibiting cell division.
This mechanism can also affect mammalian cell replication. In particular, some congeners of this drug family (for example those that contain the C-8 fluorine),[18] display high activity not only against bacterial topoisomerases, but also against eukaryotic topoisomerases and are toxic to cultured mammalian cells and in vivo tumor models.[19] Although quinolones are highly toxic to mammalian cells in culture, its mechanism of cytotoxic action is not known. Quinolone induced DNA damage was first reported in 1986 (Hussy et al.).[20]
Recent studies have demonstrated a correlation between mammalian cell cytotoxicity of the quinolones and the induction of micronuclei.[21][22][23][24] As such some fluoroquinolones, including Norfloxacin, may cause injury to the chromosome of eukaryotic cells.[25][26][27][28][29][30]
There continues to be considerable debate as to whether or not this DNA damage is to be considered one of the mechanisms of action concerning the severe adverse reactions experienced by some patients following fluoroquinolone therapy.[19][31][32]
Contraindications
As noted above, under licensed use, norfloxacin is also now considered to be contraindicated for the treatment of certain sexually transmitted diseases by some experts due to bacterial resistance.[13]
Norfloxacin is contraindicated in those with a history of tendonitis, tendon rupture and those with a hypersensitivity to fluoroquinolones.[33]
There are three contraindications found within the 2008 package[5] insert:
- ”Noroxin (norfloxacin) is contraindicated in persons with a history of hypersensitivity, tendinitis, or tendon rupture associated with the use of norfloxacin or any member of the quinolone group of antimicrobial agents.”
- ”Quinolones, including norfloxacin, have been shown in vitro to inhibit CYP1A2. Concomitant use with drugs metabolized by CYP1A2 (e.g., caffeine, clozapine, ropinirole, tacrine, theophylline, tizanidine) may result in increased substrate drug concentrations when given in usual doses. Patients taking any of these drugs concomitantly with norfloxacin should be carefully monitored.”
- “Concomitant administration with tizanidine is contraindicated”
Norfloxacin is also considered to be contraindicated within the pediatric population.
- Pregnancy
Norfloxacin has been reported to rapidly cross the blood-placenta and blood-milk barrier, and is extensively distributed into the fetal tissues.[34] For this reason norfloxacin and other fluoroquinolones are contraindicated during pregnancy due to the risk of spontaneous abortions and birth defects. The fluoroquinolones have also been reported as being present in the mother’s milk and are passed on to the nursing child, which may increases the risk of the child suffering an adverse reaction even though the child had never been prescribed or taken any of the drugs found within this class.[35][36] As safer alternatives are generally available norfloxacin is contraindicated during pregnancy, especially during the first trimester. The manufacturer only recommends use of norfloxacin during pregnancy when benefit outweighs risk.[37]
- Pediatric population
A 1998 retrospective survey found that that numerous side effects have been recorded in reference to the unapproved use of norfloxacin in the pediatric population.[38] Fluoroquinolones are not licensed by the FDA for use in children due to the risk of fatalities[39] as well as permanent injury to the musculoskeletal system, with two exceptions. Ciprofloxacin is being licensed for the treatment of Complicated Urinary Tract Infections and Pyelonephritis due to Escherichia coli and Inhalational Anthrax (post-exposure) and levofloxacin was recently licensed for the treatment of Inhalational Anthrax (post-exposure). However, the Fluoroquinolones are licensed to treat lower respiratory infections in children with cystic fibrosis in the UK.
Adverse effects
See also: Adverse effects of fluoroquinolonesSerious adverse events occur more commonly with fluoroquinolones than with any other antibiotic drug classes.[40][41]
Joint and tendon problems, as seen with all drugs within this class, have been associated with norfloxacin since 1983.[42] In 1989 Jeandel et al. comments on arthritis being induced by norfloxacin.[43] And in 1995 Terry el reports upon arthalgia being induced by norfloxacin.[44] Within the cases of tendinopathy reported to the FDA from 1987–1997, more reports of ruptures and tendonitis were associated with norfloxacin, than any other fluoroquinolone in use at that time.[45]
Hypersensitivity reactions such as erythema multiforme, TEN (toxic epidermal necrolysis),[46] Sweet syndrome (acute neutrophilic dermatosis),[47] fixed drug eruptions(FDE),[48] systemic contact dermatitis,[49] acantholytic bullous eruptions[50] as well as pustular eruptions have been associated with norfloxacin since it’s introduction.
On September 23, 2008, the FDA required the manufacturer to add an additional warning to the package inserts that stated that “Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving quinolone therapy, including Noroxin.”[51]
As with other drugs in this class norfloxacin is also associated with severe and even fatal liver diseases.[51] Acute liver failure or serious liver injury (Hepatitis), was reported in 1993.[52] Hepatitis, jaundice, including cholestatic jaundice and elevated liver function tests have been reported during norfloxacin therapy. Severe reversible thrombopenia has occurred with norfloxacin.[53] Norfloxacin-induced hepatitis,[52][54][55][56][57][58]
Acute pancreatitis has also been linked to norfloxacin.[59] Which is but one of the many serious adverse effects that may occur as a result of norfloxacin therapy. Another such serious reaction is irreversible peripheral neuropathy. In a 1996 study it was noted that paraesthesia of the feet, legs, hands and arms occurred in 81% of the patients while 51% involved reports of hypoaesthesia and numbness. Of the thirty- seven reports found within this study, thirty one involved Norfloxacin. Twenty nine percent of these patients were reported to still be experiencing peripheral sensory disturbances after therapy had been discontinued.[60]
Allergic nephropathy is also associated with norfloxacin as well other serious kidney problems.[61][62] Renal failure was first report in 1986,[63] and reports of neutropenia,[64] thrombopenia,[65][66] agranulocytosis,[67] nephrotic syndrome,[68][69] eosinophilia[70] and acute interstitial nephritis[71][72] all being associated with norfloxacin therapy. Additional serious adverse reactions include temporary as well as permanent loss of vision,[73] QTc prolongation/torsades de pointes, severe central nervous system disorders (CNS) including seizures,[74] drug induced psychosis[75] and hallucinations,[76] clostridium difficile associated disease (CDAD: Pseudomembranous colitis),[77][78][79] as well as photosensitivity/phototoxicity reactions. Pseudotumor cerebri, commonly known as idiopathic intracranial hypertension (IIH), (also referred to as increased intracranial pressure), has been reported to occur as a serious adverse reaction to norfloxacin.
Children and the elderly are at a much greater risk of experiencing such adverse reactions.[40][41] This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.[80] Such reactions may manifest during, as well as long after fluoroquinolone therapy had been discontinued.[81] Norfloxacin may also exacerbate the signs of myasthenia gravis and lead to life threatening weakness of the respiratory muscles.
Serious visual complications have also been reported to occur with ophthalmic fluoroquinolone therapy, which may also occur with norfloxacin eye drops, especially corneal perforation, but also evisceration and enucleation.[82][83][84] This increased incidents of corneal perforation may be due to fluoroquinolones causing alterations in stromal collagen, leading to a reduction in tectonic strength.[85][86] There have also been a number of reports over the years of norfloxacin deposits on the corneal causing serious vision problems following the use of eye drops.[83][84][87]
Some groups refer to these adverse events as "fluoroquinolone toxicity". These groups of people claim to have suffered serious long term harm to their health from using fluoroquinolones. This has led to a class action lawsuit by people harmed by the use of fluoroquinolones as well as legal action by the consumer advocate group Public Citizen.[88] Partly as a result of the efforts of The State of Illinois and Public Citizen the FDA ordered a black box warnings on all fluoroquinolones advising consumers of the possible toxic effects of fluoroquinolones on tendons.[89]
History of the black box warnings
Musculoskeletal disorders attributed to use of quinolone antibiotics were first reported in the medical literature in 1972, as an adverse reaction to nalidixic acid.[90] Rheumatic disease after use of a fluoroquinolone (norfloxacin) was first reported eleven years later.[91] In a 1995 letter published in the New England Journal of Medicine, representatives of the U.S. Food and Drug Administration (FDA) stated that the agency would "update the labeling [package insert] for all marketed fluoroquinolones to include a warning about the possibility of tendon rupture."[92]
By August 1996, the FDA had not taken action, and the consumer advocacy group Public Citizen filed a petition with the FDA prompting the agency to act.[93] Two months later, the FDA published an alert in the FDA Medical Bulletin and requested that fluoroquinolone package inserts be amended to include information on this risk.[94]
In 2005, the Illinois Attorney General filed a petition with the FDA seeking black box warnings and "Dear Doctor" letters emphasizing the risk of tendon rupture; the FDA responded that it had not yet been able to reach a decision on the matter.[95] In 2006, Public Citizen, supported by the Illinois Attorney General, renewed its demand of ten years prior for a black box warning.[95][96] In January 2008, Public Citizen filed suit to compel the FDA to respond to their 2006 petition.[97][98] On July 7, the FDA ordered the makers of systemic-use fluoroquinolones to add a boxed warning regarding tendon rupture, and to develop a Medication Guide for patients.[99][100] The package inserts for Cipro (ciprofloxacin), Avelox (moxifloxacin), Proquin XR, Factive (gemifloxacin), Floxin (ofloxacin), Noroxin (norfloxacin) and Levaquin (levofloxacin) were amended on September 8, 2008 to include these new warnings.[101] Bayer, which manufactures Cipro, Avelox and Proquin XR, issued a Dear Healthcare Professional letter on October 22 concerning these changes.[102] Ortho-McNeil, the manufacturers of Levaquin, issued a similar letter in November.[103] through the Health Care Notification Network, a registration-only website that distributes drug alerts to licensed healthcare professionals. To date no such letters have been issued by the manufacturers of Noroxin or other products containing norfloxacin.
Regulatory actions
There has been a significant number of regulatory actions taken as a result of such adverse reactions, which included published warnings,[104][105] additional warnings and safety information added to the package inserts[106] together with the request for the issuance of "Dear Doctor Letters" concerning the recent addition of Black Box Warnings in 2008. Although requested to do so by the FDA back in 2008, the manufacturers of Noroxin have not issued any "Dear Doctor Letters" regarding the tendon issues as of September 2009. Fifteen years ago, in 1994, the manufacturers had added minimal warnings concerning the association with norfloxacin and musculoskeletal tendinitis as well as spontaneous tendon ruptures. This change was approved by the FDA on July 1, 1994 and included minimal warnings regarding ruptures, as well as pseudomembranous colitis.[107] The tendon warnings were once again revised in 1996 as the previous warnings concerning tendon issues were considered by the FDA to be inadequate.[108]
In 2004 the FDA requested new warning labels to be added to all of the Fluoroquinolones, including norfloxacin, regarding Peripheral Neuropathy (irreversible nerve damage), Tendon Damage, Heart Problems (prolonged QT Interval / Torsades de pointes), Pseudomembranous colitis, Rhabdomyolysis (muscle wasting), Steven Johnson Syndrome, as well as concurrent usage of NSAIDs contributing to the severity of these reactions.[14] The package insert was not changed to include any warnings regarding Steven Johnson Syndrome until September, 2008, four years later. In 1994 the product information for norfloxacin was amended in Japan (October 1994), to state that rhabdomyolysis may occur.[109] A number of case reports concerning this had also been published as early as 1992.[110] This has also been reported again within the literature in 1996.[111] However the warnings concerning Rhabdomyolysis (muscle wasting) remain absent from the package inserts (as of September 2009) within the United States.
On September 23, 2008, the FDA required the manufacturer to add an additional warning to the package inserts that stated that “Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving quinolone therapy, including Noroxin.”[51]
Interactions
The toxicity of drugs that are metabolised by the cytochrome P450 system is enhanced by concomitant use of some quinolones. Quinolones, including norfloxacin, may enhance the effects of oral anticoagulants, including warfarin or its derivatives or similar agents. When these products are administered concomitantly, prothrombin time or other suitable coagulation tests should be closely monitored. Coadministration may dangerously increase coumadin warfarin activity; INR should be monitored closely. [14]
They may also interact with the GABA A receptor and cause neurological symptoms; this effect is augmented by certain non-steroidal anti-inflammatory drugs.[112] The concomitant administration of a non-steroidal anti-inflammatory drug (NSAID) with a quinolone, including norfloxacin, may increase the risk of CNS stimulation and convulsive seizures. Therefore, norfloxacin should be used with caution in individuals receiving NSAIDS concomitantly.[113]
Elevated serum levels of cyclosporine have been reported with concomitant use of cyclosporine with norfloxacin. Therefore, cyclosporine serum levels should be monitored and appropriate cyclosporine dosage adjustments made when these drugs are used concomitantly.
The concomitant administration of quinolones including norfloxacin with glyburide (a sulfonylurea agent) has, on rare occasions, resulted in severe hypoglycemia. Therefore, monitoring of blood glucose is recommended when these agents are co-administered.
Significant drug interactions
Some quinolones exert an inhibitory effect on the cytochrome P-450 system, thereby reducing theophylline clearance and increasing theophylline blood levels. Coadministration of certain fluoroquinolones and other drugs primarily metabolized by CYP1A2 (e.g. theophylline, methylxanthines, tizanidine) results in increased plasma concentrations and could lead to clinically significant side effects of the coadministered drug. Additionally other fluoroquinolones, especially enoxacin, and to a lesser extent ciprofloxacin and pefloxacin, also inhibit the metabolic clearance of theophylline.[114]
Such drug interactions are associated with the molecular structural modifications of the quinolone ring, specifically interactions involving NSAIDS and theophylline. As such, these drug interactions involving the fluoroquinolones appear to be drug specific rather than a class effect. The fluoroquinolones have also been shown to interfere with the metabolism of caffeine[115] and the absorption of levothyroxine. The interference with the metabolism of caffeine may lead to the reduced clearance of caffeine and a prolongation of its serum half-life, resulting in a caffeine overdose. This may lead to reduced clearance of caffeine and a prolongation of the plasma's half-life that may lead to accumulation of caffeine in plasma when products containing caffeine are consumed while taking norfloxacin. [14]
The use of NSAIDs (Non Steroid Anti Inflammatory Drugs) while undergoing fluoroquinolone therapy is contra-indicated due to the risk of severe CNS adverse reactions, including but not limited to seizure disorders. Fluoroquinolones with an unsubstituted piperazinyl moiety at position 7 have the potential to interact with NSAIDs and/or their metabolites, resulting in antagonism of GABA neurotransmission.[116]
The use of norfloxacin concomitantly has also been associated with transient elevations in serum creatinine in patients receiving cyclosporine, on rare occasions, resulted in severe hypoglycemia with sulfonylurea. Renal tubular transport of methotrexate may be inhibited by concomitant administration of norfloxacin, potentially leading to increased plasma levels of methotrexate. This might increase the risk of methotrexate toxic reactions.
Current or past treatment with oral corticosteroids is associated with an increased risk of Achilles tendon rupture, especially in elderly patients who are also taking the fluoroquinolones.[117]
Overdose
Treatment of overdose includes emptying of the stomach via induced vomiting or by gastric lavage. Careful monitoring and supportive treatment, monitoring of renal and liver function, and maintaining adequate hydration is recommended by the manufacturer. Administration of magnesium, aluminum, or calcium containing antacids can reduce the absorption of norfloxacin.[5]
Chemistry
"Norfloxacin, a fluoroquinolone, is a 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3quinolinecarboxylic acid. Its empirical formula is C16H18FN3O3. Norfloxacin is a white to pale yellow crystalline powder with a molecular weight of 319.34 and a melting point of about 221°C. It is freely soluble in glacial acetic acid, and very slightly soluble in ethanol, methanol and water. Norfloxacin differs from non-fluorinated quinolones by having a fluorine atom at the 6 position and a piperazine moiety at the 7 position." Quoting from the 2009 package insert for Noroxin.[5]
Pharmacokinetics
“Absorption of norfloxacin is rapid following single doses of 200 mg, 400 mg and 800 mg. At the respective doses, mean peak serum and plasma concentrations of 0.8, 1.5 and 2.4 μg/mL are attained approximately one hour after dosing. The effective half-life of norfloxacin in serum and plasma is 3–4 hours. Steady-state concentrations of norfloxacin will be attained within two days of dosing. Renal excretion occurs by both glomerular filtration and tubular secretion as evidenced by the high rate of renal clearance (approximately 275 mL/min). Within 24 hours of drug administration, 26 to 32% of the administered dose is recovered in the urine as norfloxacin with an additional 5-8% being recovered in the urine as six active metabolites of lesser antimicrobial potency. Only a small percentage (less than 1%) of the dose is recovered thereafter. Fecal recovery accounts for another 30% of the administered dose. Two to three hours after a single 400-mg dose, urinary concentrations of 200 μg/mL or more are attained in the urine. In healthy volunteers, mean urinary concentrations of norfloxacin remain above 30 μg/mL for at least 12 hours following a 400-mg dose. The urinary pH may affect the solubility of norfloxacin. Norfloxacin is least soluble at urinary pH of 7.5 with greater solubility occurring at pHs above and below this value. The serum protein binding of norfloxacin is between 10 and 15%.” Quoting from the 2009 package insert for Noroxin.[5]
Biotransformation is via the liver and kidneys, with a half-life of 3–4 hours.[8]
Dosing
The status of the patient’s renal function and hepatic function should also be taken into consideration to avoid an accumulation that may lead to a fatal drug overdose. Norfloxacin is eliminated primarily by renal excretion. However, the drug is also metabolized and partially cleared through the liver and the intestine. Modification of the dosage is recommended using the table found within the package insert for those with impaired liver or kidney function. (Particularly for patients with severe renal dysfunction.) However, since the drug is known to be substantially excreted by the kidneys, the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Additional caution is warranted in the elderly population as well. The duration of treatment depends upon the severity of infection and the usual duration is anywhere from 3 to 28 days.[5]
Susceptible bacteria
Gram-positive aerobes:
- Enterococcus faecalis
- Staphylococcus aureus
- Staphylococcus epidermidis
- Staphylococcus saprophyticus
- Streptococcus agalactiae
Gram-negative aerobes:
- Citrobacter freundii
- Enterobacter aerogenes
- Enterobacter cloacae
- Escherichia coli
- Klebsiella pneumoniae
- Neisseria gonorrhoeae
- Proteus mirabilis
- Proteus vulgaris
- Pseudomonas aeruginosa
- Serratia marcescens
As with other drugs in this class, some strains of Pseudomonas aeruginosa may develop resistance fairly rapidly during treatment with Norfloxacin.
References
- ^ Nelson, JM.; Chiller, TM.; Powers, JH.; Angulo, FJ. (Apr 2007). "Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story.". Clin Infect Dis 44 (7): 977–80. doi:10.1086/512369. PMID 17342653.
- ^ Padeĭskaia, EN. (2003). "[Norfloxacin: more than 20 years of clinical use, the results and place among fluoroquinolones in modern chemotherapy for infections]". Antibiot Khimioter 48 (9): 28–36. PMID 15002177.
- ^ Rafalsky, V.; Andreeva, I.; Rjabkova, E.; Rafalsky, Vladimir V (2006). "Quinolones for uncomplicated acute cystitis in women.". Cochrane Database Syst Rev 3: CD003597. doi:10.1002/14651858.CD003597.pub2. PMID 16856014.
- ^ "HQ 545710". faqs.org. 30 October 1998. http://www.faqs.org/rulings/rulings1999HQ545710.html.
- ^ a b c d e f g Merck Sharp & Dohme (September 2008). "TABLETS NOROXIN® (NORFLOXACIN)" (PDF). USA: FDA. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019384s052lbl.pdf.
- ^ a b c d "EMEA Restricts Use of Oral Norfloxacin Drugs in UTIs". UK: DGNews. 24 July 2008. http://www.docguide.com/news/content.nsf/news/852571020057CCF68525749000687709.
- ^ a b Merck Sharp & Dohme (September 2000). "Chibroxin (Norfloxacin) Ophthalmic solution" (PDF). USA: FDA. http://www.accessdata.fda.gov/drugsatfda_docs/label/2001/19757S10lbl.pdf.
- ^ a b "Showing drug card for Norfloxacin (DB01059)". Canada: DrugBank.ca. 19 February. http://www.drugbank.ca/drugs/DB01059.
- ^ http://sciencelinks.jp/j-east/article/200109/000020010901A0173889.php
- ^ http://www.baytril.com/index.php/fuseaction/download/lrn_file/baytril-history_090112.pdf
- ^ http://www.uspto.gov/web/offices/pac/dapp/opla/term/certs/4146719.pdf
- ^ http://prod.hopkins-abxguide.org/literature_review/09-2007/update_to_cdc_s_sexually_transmitted_diseases_treatment.html?contentInstanceId=254789&siteId=153
- ^ a b Susan Blank; Julia Schillinger (14 May 2004). "DOHMH ALERT #8:Fluoroquinolone-resistant gonorrhea, NYC". USA: New York County Medical Society. http://www.nycms.org/article_view.php3?view=947&part=1. Retrieved 22 July 2009.
- ^ a b c d http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2004/19384s040,042,043ltr.pdf
- ^ Thaver, D.; Zaidi, AK.; Critchley, JA.; Azmatullah, A.; Madni, SA.; Bhutta, ZA.; Bhutta, Zulfiqar A (2008). "Fluoroquinolones for treating typhoid and paratyphoid fever (enteric fever).". Cochrane Database Syst Rev (4): CD004530. doi:10.1002/14651858.CD004530.pub3. PMID 18843659.
- ^ Clodagh Sheehy (2 August 2008). "Warning over two types of antibiotic". Republic of Ireland. http://www.herald.ie/national-news/warning-over-two-types-of-antibiotic-1445498.html. Retrieved 17 July 2009.
- ^ Drlica K, Zhao X (1 September 1997). "DNA gyrase, topoisomerase IV, and the 4-quinolones". Microbiol Mol Biol Rev. 61 (3): 377–92. PMC 232616. PMID 9293187. http://mmbr.asm.org/cgi/pmidlookup?view=long&pmid=9293187.
- ^ Robinson MJ, Martin BA, Gootz TD, McGuirk PR, Osheroff N (April 1992). "Effects of novel fluoroquinolones on the catalytic activities of eukaryotic topoisomerase II: Influence of the C-8 fluorine group" (PDF). Antimicrob. Agents Chemother. 36 (4): 751–6. PMC 189387. PMID 1323952. http://aac.asm.org/cgi/reprint/36/4/751.pdf.
- ^ a b Sissi C, Palumbo M (November 2003). "The quinolone family: from antibacterial to anticancer agents". Curr Med Chem Anticancer Agents 3 (6): 439–50. doi:10.2174/1568011033482279. PMID 14529452. http://openurl.ingenta.com/content/nlm?genre=article&issn=1568-0118&volume=3&issue=6&spage=439&aulast=Sissi. "The present review focuses on the structural modifications responsible for the transformation of an antibacterial into an anticancer agent. Indeed, a distinctive feature of drugs based on the quinolone structure is their remarkable ability to target different type II topoisomerase enzymes. In particular, some congeners of this drug family display high activity not only against bacterial topoisomerases, but also against eukaryotic topoisomerases and are toxic to cultured mammalian cells and in vivo tumor models."
- ^ Hussy P, Maass G, Tümmler B, Grosse F, Schomburg U (June 1986). "Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts". Antimicrob. Agents Chemother. 29 (6): 1073–8. PMC 180502. PMID 3015015. http://aac.asm.org/cgi/reprint/29/6/1073.pdf.
- ^ Hosomi JA, Maeda Y, Oomori T, Irikura and T. Yokota (1988). "Mutagenicity of norfloxacin and AM-833 in bacteria and mammalian cells". Rev. Infect. Dis 10 (Suppl. 1): S148–9.
- ^ Forsgren A, Bredberg A, Pardee AB, Schlossman SF, Tedder TF (May 1987). "Effects of ciprofloxacin on eucaryotic pyrimidine nucleotide biosynthesis and cell growth". Antimicrob. Agents Chemother. 31 (5): 774–9. PMC 174831. PMID 3606077. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=3606077.
- ^ Gootz TD, Barrett JF, Sutcliffe JA (January 1990). "Inhibitory effects of quinolone antibacterial agents on eucaryotic topoisomerases and related test systems". Antimicrob. Agents Chemother. 34 (1): 8–12. PMC 171510. PMID 2158274. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=171510.
- ^ Lawrence JW, Darkin-Rattray S, Xie F, Neims AH, Rowe TC (February 1993). "4-Quinolones cause a selective loss of mitochondrial DNA from mouse L1210 leukemia cells". J. Cell. Biochem. 51 (2): 165–74. doi:10.1002/jcb.240510208. PMID 8440750.
- ^ Elsea SH, Osheroff N, Nitiss JL (July 1992). "Cytotoxicity of quinolones toward eukaryotic cells. Identification of topoisomerase II as the primary cellular target for the quinolone CP-115,953 in yeast". J. Biol. Chem. 267 (19): 13150–3. PMID 1320012. http://www.jbc.org/cgi/reprint/267/19/13150.
- ^ Suto MJ, Domagala JM, Roland GE, Mailloux GB, Cohen MA (December 1992). "Fluoroquinolones: relationships between structural variations, mammalian cell cytotoxicity, and antimicrobial activity". J. Med. Chem. 35 (25): 4745–50. doi:10.1021/jm00103a013. PMID 1469702.
- ^ Enzmann H, Wiemann C, Ahr HJ, Schlüter G (April 1999). "Damage to mitochondrial DNA induced by the quinolone Bay y 3118 in embryonic turkey liver". Mutat. Res. 425 (2): 213–24. doi:10.1016/S0027-5107(99)00044-5. PMID 10216214.
- ^ Kashida Y, Sasaki YF, Ohsawa K, et al. (October 2002). "Mechanistic study on flumequine hepatocarcinogenicity focusing on DNA damage in mice". Toxicol. Sci. 69 (2): 317–21. doi:10.1093/toxsci/69.2.317. PMID 12377980. http://toxsci.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=12377980.
- ^ Thomas A, Tocher J, Edwards DI (May 1990). "Electrochemical characteristics of five quinolone drugs and their effect on DNA damage and repair in Escherichia coli". J. Antimicrob. Chemother. 25 (5): 733–44. doi:10.1093/jac/25.5.733. PMID 2165050. http://jac.oxfordjournals.org/cgi/reprint/25/5/733.
- ^ "Fluoroquinolones and Quinolones". The American Academy of Optometry (British Chapter). http://www.academy.org.uk/pharmacy/fluoroq.htm. Retrieved 29 January 2009.
- ^ Yaseen A. Al-Soud; Najim A. Al-Masoudi (2003). "A new class of dihaloquinolones bearing N'-aldehydoglycosylhydrazides, mercapto-1,2,4-triazole, oxadiazoline and a-amino ester precursors: synthesis and antimicrobial activity". J. Braz. Chem. Soc 14 (5). doi:10.1590/S0103-50532003000500014. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-50532003000500014&lng=es&nrm=iso&tlng=es. "Nevertheless, some quinolones cause injury to the chromosome of eukaryotic cells.21,22 These findings prompted us to optimize the substituent at C-3, by..."
- ^ Yaseen A. Al-Soud a and Najim A. Al-Masoudi (2003). "A New Class of Dihaloquinolones Bearing N’-Aldehydoglycosylhydrazides, Mercapto-1,2,4-triazole, Oxadiazoline and α-Amino Ester Precursors: Synthesis and Antimicrobial Activity". J. Braz. Chem. Soc 14 (5): 790–796. doi:10.1590/S0103-50532003000500014. http://jbcs.sbq.org.br/jbcs/2003/v14_n5/13-048-02.pdf. "Although the current quinolones are not considered to be potent inhibitors of eucaryotic topoisomerases, some effects on these and other enzymes involved with DNA replication have been observed"
- ^ "19-384/S027" (PDF). USA: FDA. 1995. http://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/019384_S027_NOROXIN.pdf.
- ^ http://drugsafetysite.com/norfloxacin/ referencing: T.P. Dowling, personal communication, Merck & Co, Inc., 1987
- ^ Shin HC, Kim JC, Chung MK, et al., HC (September 2003). "Fetal and maternal tissue distribution of the new fluoroquinolone DW-116 in pregnant rats". Comp. Biochem. Physiol. C Toxicol. Pharmacol. 136 (1): 95–102. doi:10.1016/j.cca.2003.08.004. ISSN 1532-0456. PMID 14522602.
- ^ Dan M, Weidekamm E, Sagiv R, Portmann R, Zakut H, M (February 1993). "Penetration of fleroxacin into breast milk and pharmacokinetics in lactating women". Antimicrob. Agents Chemother. 37 (2): 293–6. ISSN 0066-4804. PMC 187655. PMID 8452360. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=8452360.
- ^ http://www.drugs.com/pregnancy/norfloxacin.html
- ^ Pariente-Khayat, A.; Vauzelle-Kervroedan, F.; d'Athis, P.; Bréart, G.; Gendrel, D.; Aujard, Y.; Olive, G.; Pons, G. (May 1998). "[Retrospective survey of fluoroquinolone use in children]". Arch Pediatr 5 (5): 484–8. doi:10.1016/S0929-693X(99)80311-X. PMID 9759180.
- ^ Karande SC, Kshirsagar NA, SC (February 1992). "Adverse drug reaction monitoring of ciprofloxacin in pediatric practice" (Free full text). Indian Pediatr 29 (2): 181–8. ISSN 0019-6061. PMID 1592498.
- ^ a b Owens, Rc RC Jr; Ambrose, PG (July 2005). "Antimicrobial safety: focus on fluoroquinolones.". Clinical Infectious Diseases 41 (Suppl 2): S144–57. doi:10.1086/428055. ISSN 1058-4838. PMID 15942881.
- ^ a b Iannini, PB (June 2007). "The safety profile of moxifloxacin and other fluoroquinolones in special patient populations.". Current medical research and opinion 23 (6): 1403–13. doi:10.1185/030079907X188099. ISSN 0300-7995. PMID 17559736.
- ^ Bailey, RR.; Kirk, JA.; Peddie, BA. (July 1983). "Norfloxacin-induced rheumatic disease.". N Z Med J 96 (736): 590. PMID 6223241.
- ^ Jeandel, C.; Manciaux, MA.; Bannwarth, B.; Pere, P.; Penin, F.; Netter, P.; Cuny, G. (April 1989). "Arthritis induced by norfloxacin.". J Rheumatol 16 (4): 560–1. PMID 2746601.
- ^ Terry, JB. (April 1995). "Norfloxacin induced arthralgia.". J Rheumatol 22 (4): 793–4. PMID 7791191.
- ^ Harrell RM (June 1999). "Fluoroquinolone-induced tendinopathy: what do we know?". South. Med. J. 92 (6): 622–5. doi:10.1097/00007611-199906000-00014. PMID 10372859.
- ^ Sahin, MT.; Ozturkcan, S.; Inanir, I.; Filiz, EE. (April 2005). "Norfloxacin-induced toxic epidermal necrolysis.". Ann Pharmacother 39 (4): 768–70. doi:10.1345/aph.1E530. PMID 15713789.
- ^ Aguiar-Bujanda, D.; Aguiar-Morales, J.; Bohn-Sarmiento, U. (January 2004). "Sweet's syndrome associated with norfloxacin in a prostate cancer patient.". QJM 97 (1): 55–6. doi:10.1093/qjmed/hch011. PMID 14702513.
- ^ Fernandez-Rivas, M. (April 1997). "Fixed drug eruption (FDE) caused by norfloxacin.". Allergy 52 (4): 477–8. doi:10.1111/j.1398-9995.1997.tb01035.x. PMID 9188937.
- ^ Silvestre, JF.; Alfonso, R.; Moragón, M.; Ramón, R.; Botella, R. (August 1998). "Systemic contact dermatitis due to norfloxacin with a positive patch test to quinoline mix.". Contact Dermatitis 39 (2): 83. doi:10.1111/j.1600-0536.1998.tb05839.x. PMID 9746189.
- ^ Ramsay, B.; Woodrow, D.; Cream, JJ. (October 1993). "An acantholytic bullous eruption after norfloxacin.". Br J Dermatol 129 (4): 500. doi:10.1111/j.1365-2133.1993.tb03185.x. PMID 8217768.
- ^ a b c http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2008/019384s049ltr.pdf
- ^ a b Davoren, P.; Mainstone, K. (September 1993). "Norfloxacin-induced hepatitis.". Med J Aust 159 (6): 423, 426. PMID 8377697.
- ^ Chamouard, P.; Duclos, B.; Welsch, M.; Gold, A. (November 1987). "[Severe reversible thrombopenia induced by norfloxacin]". Presse Med 16 (39): 1978–9. PMID 2962165.
- ^ Lucena, MI.; Andrade, RJ.; Sanchez-Martinez, H.; Perez-Serrano, JM.; Gomez-Outes, A. (November 1998). "Norfloxacin-induced cholestatic jaundice.". Am J Gastroenterol 93 (11): 2309–11. doi:10.1111/j.1572-0241.1998.02309.x. PMID 9820434.
- ^ Romero-Gómez, M.; Suárez García, E.; Fernández, MC. (August 1999). "Norfloxacin-induced acute cholestatic hepatitis in a patient with alcoholic liver cirrhosis.". Am J Gastroenterol 94 (8): 2324–5. doi:10.1111/j.1572-0241.1999.02324.x. PMID 10445586.
- ^ Abad, A.; López, P.; Bauza, J. (1999). "Norfloxacin-induced positive direct antiglobulin test.". Vox Sang 77 (4): 238. doi:10.1046/j.1423-0410.1999.7740238.x. PMID 10717605.
- ^ Björnsson, E.; Olsson, R.; Remotti, H. (December 2000). "Norfloxacin-induced eosinophilic necrotizing granulomatous hepatitis.". Am J Gastroenterol 95 (12): 3662–4. doi:10.1111/j.1572-0241.2000.03404.x. PMID 11151924.
- ^ Coleman, CI.; Spencer, JV.; Chung, JO.; Reddy, P. (July–August 2002). "Possible gatifloxacin-induced fulminant hepatic failure.". Ann Pharmacother 36 (7-8): 1162–7. doi:10.1345/aph.1A414. PMID 12086547. "Fluoroquinolones, including trovafloxacin, ciprofloxacin, ofloxacin, enoxacin, norfloxacin, and, in this case report, gatifloxacin, have been associated with hepatotoxicity."
- ^ Drabo, YJ.; Niakara, A.; Ouedraogo, H. (January 2002). "[Acute pancreatitis secondary to administration or norfloxacin]". Ann Fr Anesth Reanim 21 (1): 68–9. PMID 11878127.
- ^ Hedenmalm, K.; Spigset, O. (April 1996). "Peripheral sensory disturbances related to treatment with fluoroquinolones.". J Antimicrob Chemother 37 (4): 831–7. doi:10.1093/jac/37.4.831. PMID 8722551. http://www.fqresearch.org/pdf_files/84.pdf.
- ^ Hadimeri, H.; Almroth, G.; Cederbrant, K.; Eneström, S.; Hultman, P.; Lindell, A. (October 1997). "Allergic nephropathy associated with norfloxacin and ciprofloxacin therapy. Report of two cases and review of the literature.". Scand J Urol Nephrol 31 (5): 481–5. doi:10.3109/00365599709030647. PMID 9406312.
- ^ Green, L.; Clark, J. (November 1989). "Fluoroquinolones and theophylline toxicity: norfloxacin.". JAMA 262 (17): 2383. doi:10.1001/jama.262.17.2383b. PMID 2795820.
- ^ Boelaert, J.; de Jaegere, PP.; Daneels, R.; Schurgers, M.; Gordts, B.; van Landuyt, HW. (May 1986). "Case report of renal failure during norfloxacin therapy.". Clin Nephrol 25 (5): 272. PMID 3522001.
- ^ Patoia, L.; Guerciolini, R.; Menichetti, F.; Bucaneve, G.; Del Favero, A. (November 1987). "Norfloxacin and neutropenia.". Ann Intern Med 107 (5): 788–9. PMID 3662307.
- ^ Chamouard, P.; Duclos, B.; Welsch, M.; Gold, A. (November 1987). "[Severe reversible thrombopenia induced by norfloxacin]". Presse Med 16 (39): 1978–9. PMID 2962165.
- ^ Wensing, JW.; Vlasveld, LT. (August 1997). "[Immune thrombocytopenia attributed to norfloxacin]". Ned Tijdschr Geneeskd 141 (34): 1660–2. PMID 9543779.
- ^ Urquia, A.; Ziad, F.; Serrano, R.; Lacasa, J.; Llinares, F. (May 1989). "[Agranulocytosis caused by norfloxacin]". Enferm Infecc Microbiol Clin 7 (5): 281–2. PMID 2490728.
- ^ Hanson, B.; D'Hondt, A.; Depierreux, M.; Lustman, F. (1996). "Nephrotic syndrome after norfloxacin.". Nephron 74 (2): 446. doi:10.1159/000189360. PMID 8893181.
- ^ Hestin, D.; Hanesse, B.; Frimat, L.; Renaudin, JM.; Netter, P.; Kessler, M.; Kessler, M (March 1995). "Norfloxacin-induced nephrotic syndrome.". Lancet 345 (8951): 732–3. doi:10.1016/S0140-6736(95)90906-0. PMID 7885154.
- ^ Mofredj, A.; Boudjema, H.; Cadranel, JF. (June 2002). "Norfloxacin-induced eosinophilia in a cirrhotic patient.". Ann Pharmacother 36 (6): 1107–8. doi:10.1345/aph.1A430. PMID 12058709.
- ^ Nakamura, M.; Ohishi, A.; Aosaki, N.; Hamaguchi, K. (October 2000). "Norfloxacin-induced acute interstitial nephritis.". Nephron 86 (2): 204–5. doi:10.1159/000045749. PMID 11015000.
- ^ Cuxart, M.; Picazo, M.; Sans, R.; Muntané, MJ. (2007). "[Norfloxacin-induced acute interstitial nephritis"]. Nefrologia 27 (5): 649. PMID 18045047. http://www.revistanefrologia.com/nlm/fichanlm.asp?id=3800.
- ^ Fraunfelder, FW; Fraunfelder, FT (September 2009). "Diplopia and fluoroquinolones". Ophthalmology 116 (9): 1814–7. doi:10.1016/j.ophtha.2009.06.027. ISSN 0161-6420. PMID 19643481.
- ^ Anastasio, GD.; Menscer, D.; Little, JM. (July 1988). "Norfloxacin and seizures.". Ann Intern Med 109 (2): 169–70. PMID 3382111.
- ^ Jain, AP.; Diwan, SK.; Chandra, K. (October 1994). "Acute psychosis with Norfloxacin.". J Assoc Physicians India 42 (10): 844. PMID 7876067.
- ^ Kundu, AK. (September 2000). "Norfloxacin-induced hallucination--an unusual CNS toxicity of 4-fluoroquinolones.". J Assoc Physicians India 48 (9): 944. PMID 11198813.
- ^ Tordjman, R.; Benfiguig, K.; Eugène, C.; Mitry, E.; Merrer, J. (May 1995). "[Pseudomembranous colitis probably caused by Clostridium difficile: first case appeared during preventive treatment of infection of ascitic fluid with norfloxacin]". Gastroenterol Clin Biol 19 (5): 545–6. PMID 7590012.
- ^ Ehrenpreis, ED.; Lievens, MW.; Craig, RM. (April 1990). "Clostridium difficile-associated diarrhea after norfloxacin.". J Clin Gastroenterol 12 (2): 188–9. doi:10.1097/00004836-199004000-00015. PMID 2324482.
- ^ Loffeld, RJ.; Flendrig, JA. (January 1990). "[Pseudomembranous colitis under administration of norfloxacin]". Ned Tijdschr Geneeskd 134 (2): 83. PMID 2296328.
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2001/19384S38ltr.pdf
- ^ Saint, F; Gueguen, G; Biserte, J; Fontaine, C; Mazeman, E (September 2000). "Rupture of the patellar ligament one month after treatment with fluoroquinolone". Revue de chirurgie orthopedique et reparatrice de l'appareil moteur 86 (5): 495–7. ISSN 0035-1040. PMID 10970974.
- ^ Shelley, ED.; Shelley, WB. (July 1988). "The subcorneal pustular drug eruption: an example induced by norfloxacin.". Cutis 42 (1): 24–7. PMID 2974410.
- ^ a b Konishi, M.; Yamada, M.; Mashima, Y. (February 1998). "Corneal ulcer associated with deposits of norfloxacin.". Am J Ophthalmol 125 (2): 258–60. doi:10.1016/S0002-9394(99)80104-4. PMID 9467459.
- ^ a b Castillo, A.; Benitez del Castillo, JM.; Toledano, N.; Diaz-Valle, D.; Sayagues, O.; Garcia-Sanchez, J. (July 1997). "Deposits of topical norfloxacin in the treatment of bacterial keratitis.". Cornea 16 (4): 420–3. doi:10.1097/00003226-199707000-00008. PMID 9220239.
- ^ Gangopadhyay, N; Daniell, M; Weih, L; Taylor, HR (April 2000). "Fluoroquinolone and fortified antibiotics for treating bacterial corneal ulcers.". The British journal of ophthalmology 84 (4): 378–84. doi:10.1136/bjo.84.4.378. ISSN 0007-1161. PMC 1723447. PMID 10729294. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1723447.
- ^ Walter, K; Tyler, ME (August 2006). "Severe corneal toxicity after topical fluoroquinolone therapy: report of two cases.". Cornea 25 (7): 855–7. doi:10.1097/01.ico.0000224642.43601.14. ISSN 0277-3740. PMID 17068466.
- ^ Parent, X.; Marchal, A.; Patillon, JC. (2005). "[Corneal precipitation of fluoroquinolones with magnesium]". Ann Biol Clin (Paris) 63 (1): 89–92. PMID 15689317.
- ^ "Public Citizen Warns of Cipro Dangers". USA: Consumer affairs. 30 August 2006. http://www.consumeraffairs.com/news04/2006/08/pubcit_cipro.html. Retrieved 7 September 2009.
- ^ "FDA orders 'black box' label on some antibiotics". CNN. 2008-07-08. http://www.cnn.com/2008/HEALTH/07/08/antibiotics.risk/index.html. Retrieved 2008-07-08.
- ^ Bailey RR, Natale R, Linton AL (October 1972). "Nalidixic acid arthralgia". Can Med Assoc J 107 (7): 604 passim. PMC 1940945. PMID 4541768. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1940945.
- ^ Bailey RR, Kirk JA, Peddie BA (July 1983). "Norfloxacin-induced rheumatic disease". N Z Med J 96 (736): 590. PMID 6223241.
- ^ Szarfman A, Chen M, Blum MD (January 1995). "More on fluoroquinolone antibiotics and tendon rupture" (letter). N Engl J Med 332 (3): 193. doi:10.1056/NEJM199501193320319. PMID 7800023.
- ^ "Petition to Require a Warning on All Fluoroquinolone Antibiotics (HRG Publication #1399)". Public Citizen. August 1, 1996. http://www.citizen.org/publications/release.cfm?ID=6595. Retrieved on December 27, 2008.
- ^ "Reports of adverse events with fluoroquinolones". FDA Medical Bulletin 26 (3). October 1996. http://www.fda.gov/medbull/oct96/adverse.html.[dead link] Retrieved on December 27, 2008. alternate link: http://www.fqresearch.org/text_documents/FDA_Medical_Bulletin_1996.doc
- ^ a b "Madigan, Public Citizen, petition FDA for "black box" warning regarding potential adverse effects of certain popular antibiotics" (Press release). Office of the Illinois Attorney General. August 29, 2006. http://www.illinoisattorneygeneral.gov/pressroom/2006_08/20060829.html. Retrieved 2008-12-27. Full text of the 2005 petition and FDA response available from the Fluoroquinolone Toxicity Research Foundation, a U.S. consumer advocacy group.
- ^ "Public Citizen Petitions the FDA to Include a Black Box Warning on Fluoroquinolone Antibiotics (HRG Publication #1781)". Public Citizen. August 29, 2006. http://www.citizen.org/publications/release.cfm?ID=7453. Retrieved 2008-12-27.
- ^ "Public Citizen v. Food and Drug Administration (FDA) (Fluoroquinolone)". Public Citizen. January 3, 2008. http://www.citizen.org/litigation/forms/cases/CaseDetails.cfm?cID=444. Retrieved 2008-12-27.
- ^ Ravn, Karen (August 18, 2008). "Behind the FDA’s ‘black box’ warnings". Los Angeles Times. http://articles.latimes.com/2008/aug/18/health/he-closer18. Retrieved 2008-12-27.
- ^ "FDA Requests Boxed Warnings on Fluoroquinolone Antimicrobial Drugs" (Press release). U.S. Food and Drug Administration. 2008-07-08. http://www.fda.gov/bbs/topics/NEWS/2008/NEW01858.html. Retrieved 2008-10-11.
- ^ http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116919.htm
- ^ The complete labeling history of each drug is available from Drugs@FDA. Medication Guides are available from the FDA's MedWatch system.
- ^ MacCarthy, Paul (October 22, 2008). "Important Change in the Avelox (moxifloxacin hydrochloride) and Cipro (ciprofloxacin) Complete Prescribing Information – Addition of Boxed Warning and Medication Guide Regarding Tendinitis and Tendon Rupture". Bayer HealthCare Pharmaceuticals. http://www.cipro.com/html/pdf/dhpl.pdf. Retrieved 2008-12-27.
- ^ Rosenthal, Norman (November 2008). "Important Change in the LEVAQUIN (Ievofloxacin) Complete Prescribing Information -Addition of Boxed Warning and Medication Guide Regarding Tendinitis and Tendon Rupture". Ortho-McNeil Janssen Scientific Affairs, LLC. http://www.fqresearch.org/pdf_files/Levaquin_11_2008_ortho_mcneil_dear_dr_letter.pdf. Retrieved 2008-12-27.
- ^ U S Food and Drug Administration (8 July 2008). "FDA Requests Boxed Warnings on Fluoroquinolone Antimicrobial Drugs" (PDF). USA: FDA. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116919.htm. Retrieved 5 September 2009.
- ^ US Food and Drug Administration (2008). "Fluoroquinolone Antimicrobial Drugs [ciprofloxacin (marketed as Cipro and generic ciprofloxacin), ciprofloxacin extended release (marketed as Cipro XR and Proquin XR), gemifloxacin (marketed as Factive), levofloxacin (marketed as Levaquin), moxifloxacin (marketed as Avelox), norfloxacin (marketed as Noroxin), and ofloxacin (marketed as Floxin and generic ofloxacin)"]. USA. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm084316.htm. Retrieved 5 September 2009.
- ^ US Food and Drug Administration. "Drugs at FDA: FDA Approved Drug Products". USA: FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist. Retrieved 5 September 2009. Noroxin as the search term
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/019384_S024_NOROXIN.pdf
- ^ "Approval package application number: 19-384" (in USA) (PDF). USA: FDA. 1996. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/019384_S029_NOROXIN.pdf.
- ^ (JPNARD) Information on Adverse Reactions to Drugs, No.128, (Oct 1994)
- ^ Kubo-Shimasaki, A.; Yoshimoto, K.; Tatsumi, E.; Yoneda, N.; Yamaguchi, N. (June 1992). "[Norfloxacin-induced infectious mononucleosis (IM)-like syndrome with Stevens-Johnson syndrome]". Rinsho Ketsueki 33 (6): 823–8. PMID 1433924.
- ^ Blain, H.; Klein, M.; Weryha, G.; Tréchot, P.; Hanesse, B.; Leclère, J. (1996). "[Rhabdomyolysis and unexplained malaise. Role of combination of ciprofibrate and norfloxacin]". Rev Med Interne 17 (10): 859–60. PMID 8976986.
- ^ Brouwers JR, JR (July 1992). "Drug interactions with quinolone antibacterials". Drug Saf 7 (4): 268–81. doi:10.2165/00002018-199207040-00003. ISSN 0114-5916. PMID 1524699.
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2006/019384s045LTR.pdf
- ^ Janknegt R, R (November 1990). "Drug interactions with quinolones". J. Antimicrob. Chemother. 26 Suppl D: 7–29. ISSN 0305-7453. PMID 2286594.
- ^ Harder S, Fuhr U, Staib AH, Wolff T, S (November 1989). "Ciprofloxacin-caffeine: a drug interaction established using in vivo and in vitro investigations" (Free full text). Am. J. Med. 87 (5A): 89S–91S. doi:10.1016/0002-9343(89)90031-4. ISSN 0002-9343. PMID 2589393. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@rn+58-08-2.
- ^ Domagala JM, JM (April 1994). "Structure-activity and structure-side-effect relationships for the quinolone antibacterials". J. Antimicrob. Chemother. 33 (4): 685–706. doi:10.1093/jac/33.4.685. ISSN 0305-7453. PMID 8056688. http://jac.oxfordjournals.org/cgi/reprint/33/4/685.
- ^ van der Linden PD, Sturkenboom MC, Herings RM, Leufkens HM, Rowlands S, Stricker BH, PD (August 2003). "Increased risk of achilles tendon rupture with quinolone antibacterial use, especially in elderly patients taking oral corticosteroids". Arch. Intern. Med. 163 (15): 1801–7. doi:10.1001/archinte.163.15.1801. ISSN 0003-9926. PMID 12912715. http://archinte.ama-assn.org/cgi/content/full/163/15/1801.
Antibacterials: nucleic acid inhibitors (J01E, J01M) Antifolates
(inhibits
purine metabolism,
thereby inhibiting
DNA and RNA synthesis)Sulfonamides
(DHPS inhibitor)Other/ungroupedCombinationsTopoisomerase
inhibitors/
quinolones/
(inhibits
DNA replication)1st g.2nd g.Ciprofloxacin# • Enoxacin‡ • Fleroxacin‡ • Lomefloxacin • Nadifloxacin • Ofloxacin • Norfloxacin • Pefloxacin • Rufloxacin3rd g.4th g.Besifloxacin • Clinafloxacin† • Garenoxacin • Gemifloxacin • Moxifloxacin • Gatifloxacin‡ • Sitafloxacin • Trovafloxacin‡/Alatrofloxacin‡ • PrulifloxacinVet.Related (DG)Anaerobic DNA
inhibitorsNitrofuran derivativesRNA synthesis Categories:- Fluoroquinolone antibiotics
- Piperazines
Wikimedia Foundation. 2010.
