- Aortic valve stenosis
-
Aortic valve stenosis Classification and external resources ICD-10 I35.0, I06.0, Q23.0 ICD-9 395.0, 396.0, 746.3 DiseasesDB 844 MedlinePlus 000178 eMedicine med/157 Aortic valve stenosis (AS) is a disease of the heart valves in which the opening of the aortic valve is narrowed.[1] The aortic valve is the valve between the left ventricle of the heart and the aorta, which is the largest artery in the body and carries the entire output of blood.
Contents
Pathophysiology
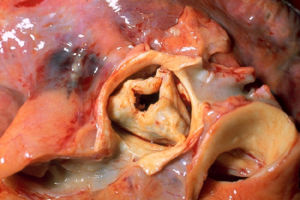 In the centre is an aortic valve with severe stenosis due to rheumatic heart disease. The valve is surrounded by the aorta. The pulmonary trunk is at the lower right. The right coronary artery, cut lengthwise, is at the lower left. The left main coronary artery, also cut lengthwise, is on the right. Autopsy specimen.
In the centre is an aortic valve with severe stenosis due to rheumatic heart disease. The valve is surrounded by the aorta. The pulmonary trunk is at the lower right. The right coronary artery, cut lengthwise, is at the lower left. The left main coronary artery, also cut lengthwise, is on the right. Autopsy specimen.
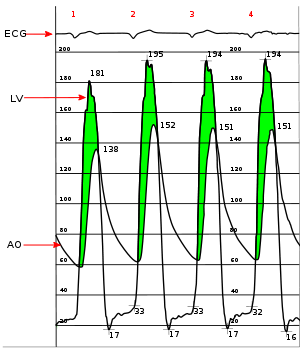 Simultaneous left ventricular and aortic pressure tracings demonstrate a pressure gradient between the left ventricle and aorta, suggesting aortic stenosis. The left ventricle generates higher pressures than what is transmitted to the aorta. The pressure gradient, caused by aortic stenosis, is represented by the green shaded area. (AO = ascending aorta; LV = left ventricle; ECG = electrocardiogram.)
Simultaneous left ventricular and aortic pressure tracings demonstrate a pressure gradient between the left ventricle and aorta, suggesting aortic stenosis. The left ventricle generates higher pressures than what is transmitted to the aorta. The pressure gradient, caused by aortic stenosis, is represented by the green shaded area. (AO = ascending aorta; LV = left ventricle; ECG = electrocardiogram.)
The aortic valve normally consists of three leaflets (trileaflets). When the left ventricle (LV) contracts, it forces blood through the valve to the aorta and then to the rest of the body. When the LV expands again, the aortic valve prevents the blood from returning to the ventricle. When the opening of the aortic valve becomes narrowed or constricted (stenotic), the blood can't be pumped adequately and the pressure in the left ventricle increases.[2] Initially, the LV compensates by thickening its walls (myocardial hypertrophy) in order to maintain adequate pumping pressure. The type of hypertrophy most commonly seen in AS is concentric hypertrophy, in which the walls of the LV are (approximately) equally thickened. In the later stages, the left ventricle dilates, the wall thins, and the systolic function deteriorates.
Etiology
Aortic stenosis is most commonly caused by age-related progressive calcification of the normal tricuspid aortic valve (>50% of cases). Other causes include calcification of a congenital bicuspid aortic valve (30-40% of cases) and acute rheumatic fever (less than 10% of cases).[3]
Normal valves have three leafs (tricuspid), but some valves have two leafs (bicuspid). Typically, aortic stenosis due to calcification of a bicuspid valve appears earlier, in the 40s and 50s, whereas that due to calcification of a normal valve appears later, in the 70s and 80s. Hypertension, diabetes mellitus, hyperlipoproteinemia and uremia may speed up the process.[3]
Prevalence
Approximately 2% of people over the age of 65, 3% of people over age 75, and 4% percent of people over age 85 have aortic valve stenosis.[4] The prevalence is increasing with the aging population in North America and Europe.[5]
Symptoms of aortic stenosis
Symptoms related to aortic stenosis depend on the degree of valve stenosis. Most people with mild to moderate aortic stenosis do not have symptoms. Symptoms usually are manifest in those with severe aortic stenosis, although they can exist in those with mild to moderate severity as well. The initial presenting symptoms include progressive shortness of breath on exertion, which may be so subtle that the the patient is unaware of them, and may cut down on exertion without being aware of his/her reduced capacity. More worrisome symptoms include syncope, chest pain, and frank heart failure.
Congestive heart failure
Congestive heart failure (CHF) carries a grave prognosis in patients with AS. Patients with CHF that is attributed to AS have a 2 year mortality rate of 50%, if the aortic valve is not replaced.
CHF in the setting of AS is due to a combination of systolic dysfunction (a decrease in the ejection fraction) and diastolic dysfunction (elevated filling pressure of the LV).
Syncope
Syncope (fainting spells) from aortic valve stenosis is usually exertional.[6] In the setting of heart failure it increases the risk of death. In patients with syncope, the 3 year mortality rate is 50%, if the aortic valve is not replaced.
It is unclear why aortic stenosis causes syncope. One popular theory is that severe AS produces a nearly fixed cardiac output. When the patient exercises, their peripheral vascular resistance will decrease as the blood vessels of the skeletal muscles dilate to allow the muscles to receive more blood to allow them to do more work. This decrease in peripheral vascular resistance is normally compensated for by an increase in the cardiac output. Since patients with severe AS cannot increase their cardiac output, the blood pressure falls and the patient will syncopize due to decreased blood perfusion to the brain.
A second theory as to why syncope may occur in AS is that during exercise, the high pressures generated in the hypertrophied LV cause a vasodepressor response, which causes a secondary peripheral vasodilation that, in turn, causes decreased blood flow to the brain. Indeed, in aortic stenosis, because of the fixed obstruction to bloodflow out from the heart, it may be impossible for the heart to increase its output to offset peripheral vasodilation.
A third mechanism may sometimes be operative. Due to the hypertrophy of the left ventricle in aortic stenosis, including the consequent inability of the coronary arteries to adequately supply blood to the myocardium (see "Angina" below), arrhythmias may develop. These can lead to syncope.
Finally, in calcific aortic stenosis at least, the calcification in and around the aortic valve can progress and extend to involve the electrical conduction system of the heart. If that occurs, the result may be heart block - a potentially lethal condition of which syncope may be a symptom.
Angina
Angina in the setting of heart failure also increases the risk of death. In patients with angina, the 5 year mortality rate is 50%, if the aortic valve is not replaced.
Angina in the setting of AS is secondary to the left ventricular hypertrophy (LVH) that is caused by the constant production of increased pressure required to overcome the pressure gradient caused by the AS. While the myocardium (i.e., heart muscle) of the LV gets thicker, the arteries that supply the muscle do not get significantly longer or bigger, so the muscle may become ischemic (i.e., does not receive an adequate blood supply). The ischemia may first be evident during exercise, when the heart muscle requires increased blood supply to compensate for the increased workload. The individual may complain of exertional angina. At this stage, a stress test with imaging may be suggestive of ischemia.
Eventually, however, the muscle will require more blood supply at rest than can be supplied by the coronary artery branches. At this point there may be signs of ventricular strain pattern (ST segment depression and T wave inversion) on the EKG, suggesting subendocardial ischemia. The subendocardium is the region that becomes ischemic because it is the most distant from the epicardial coronary arteries.
Associated symptoms
In Heyde's syndrome, aortic stenosis is associated with angiodysplasia of the colon. Recent research has shown that the stenosis causes a form of von Willebrand disease by breaking down its associated coagulation factor (factor VIII-associated antigen, also called von Willebrand factor), due to increased turbulence around the stenosed valve.
Physical examination
Aortic stenosis is most often diagnosed when it is asymptomatic and can sometimes be detected during routine examination of the heart and circulatory system. Good evidence exists to demonstrate that certain characteristics of the peripheral pulse can rule in the diagnosis.[7] In particular, there may be a slow and/or sustained upstroke of the arterial pulse, and the pulse may be of low volume. This is sometimes referred to as pulsus parvus et tardus.[3][6] There may also be a noticeable delay between the first heart sound (on auscultation) and the corresponding pulse in the carotid artery (so-called 'apical-carotid delay'). In similar manner, there may be a delay between the appearance of each pulse in the brachial artery (in the arm) and the radial artery (in the wrist).
The first heart sound may be followed by a sharp ejection sound ("ejection click") best heard at the lower left sternal border and the apex, and, thus, appear to be "split". The ejection sound, caused by the impact of left ventricular outflow against the partially fused aortic valve leaflets, is more commonly associated with a mobile bicuspid aortic valve than an immobile calcified aortic valve. The intensity of this sound does not vary with respiration, which helps distinguish it from the ejection click produced by a stenotic pulmonary valve, which will diminish slightly in intensity during inspiration.[8]
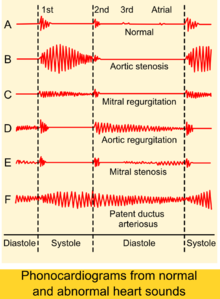
An easily heard systolic, crescendo-decrescendo (i.e., 'ejection') murmur is heard loudest at the upper right sternal border, at the 2nd right intercostal space,[3] and radiates to the carotid arteries bilaterally.[6] The murmur increases with squatting, decreases with standing and isometric muscular contraction, which helps distinguish it from hypertrophic obstructive cardiomyopathy (HOCM). The murmur is louder during expiration, but is also easily heard during inspiration. The more severe the degree of the stenosis, the later the peak occurs in the crescendo-decrescendo of the murmur.
The second heart sound (A2) tends to become decreased and softer as the aortic stenosis becomes more severe.[3] This is a result of the increasing calcification of the valve preventing it from "snapping" shut and producing a sharp, loud sound. Due to increases in left ventricular pressure from the stenotic aortic valve, over time the ventricle may hypertrophy, resulting in a diastolic dysfunction. As a result, one may hear a fourth heart sound due to the stiff ventricle.[6] With continued increases in ventricular pressure, dilatation of the ventricle will occur, and a third heart sound may be manifest.
Finally, aortic stenosis often co-exists with some degree of aortic insufficiency (aortic regurgitation). Hence, the physical exam in aortic stenosis may also reveal signs of the latter, for example an early diastolic decrescendo murmur. Indeed, when both valve abnormalities are present, the expected findings of either may be modified or may not even be present. Rather, new signs that reflect the presence of simultaneous aortic stenosis and insufficiency, e.g., pulsus bisferiens, emerge.
According to a meta analysis, the most useful findings for ruling in aortic stenosis in the clinical setting were slow rate of rise of the carotid pulse(positive likelihood ratio ranged 2.8–130 across studies), mid to late peak intensity of the murmur(positive likelihood ratio, 8.0–101), and decreased intensity of the second heart sound (positive likelihood ratio, 3.1–50).[7]
Other peripheral signs include:
- sustained, heaving apex beat,[6] which is not displaced unless systolic dysfunction of the left ventricular has developed
- A precordial thrill[6]
- narrowed pulse pressure
Diagnostic tests
The electrocardiogram (ECG)
Although aortic stenosis does not lead to any specific findings on the ECG, it still often leads to a number of electrocardiographic abnormalities. ECG manifestations of left ventricular hypertrophy (LVH) are common in aortic stenosis[6] and arise as a result of the stenosis having placed a chronically high pressure load on the left ventricle (with LVH being the expected response to chronic pressure loads on the left ventricle no matter what the cause).
As noted above, the calcification process that occurs in aortic stenosis can progress to extend beyond the aortic valve and into the electrical conduction system of the heart. Evidence of this phenomenon may include heart block that is apparent on the ECG but otherwise undetectable.
Heart catheterization
Cardiac chamber catheterization provides a definitive diagnosis, indicating severe stenosis in valve area of <0.8 cm2 (normally 1.5 to 2 cm2). It can directly measure the pressure on both sides of the aortic valve. The pressure gradient may be used as a decision point for treatment. It is useful in symptomatic patients before surgery.[6]
Echocardiogram
Severity of aortic stenosis[3] Degree of aortic stenosis Mean gradient
(mmHg)Aortic valve area
(cm2)Mild aortic stenosis <25 >1.5 Moderate aortic stenosis 25 - 40 1.0 - 1.5 Severe aortic stenosis >40 < 1.0 Critical aortic stenosis >70 < 0.6 Echocardiogram (heart ultrasound) is the best non-invasive test to evaluate the aortic valve anatomy and function.
The aortic valve area can be calculated non-invasively using echocardiographic flow velocities. Using the velocity of the blood through the valve, the pressure gradient across can be calculated by the modified Bernoulli's equation:
Gradient = 4(velocity)² mmHg
A normal aortic valve has a gradient of only a few mmHg. A decreased valvular area causes increased pressure gradient, and these parameters are used to classify aortic stenosis as either mild, moderate or severe. The pressure gradient can be abnormally low in the presence of mitral stenosis, heart failure or co-existent aortic regurgitation.
Echocardiogram may also show left ventricular hyperthrophy, thickened and immobile aortic valve and dilated aortic root.[6] However, it may appear deceptively normal in acute cases.[3]
Chest X-ray
Chest X-ray can also assist in the diagnosis, showing calcific aortic valve, and, in longstanding disease, enlarged left ventricle[3][6] and atrium.[6]
Cautions
People with aortic stenosis of any aetiology are at risk for the development of infection of their stenosed valve, i.e., infective endocarditis. To lessen the chance of developing that serious complication, people with AS are usually advised to take antibiotic prophylaxis around the time of certain dental/medical/surgical procedures. Such procedures may include dental extraction, deep scaling of the teeth, gum surgery, dental implants, treatment of esophageal varices, dilation of esophageal strictures, gastrointestinal surgery where the intestinal mucosa will be disrupted, prostate surgery, urethral stricture dilation, and cystoscopy. Note that routine upper and lower GI endoscopy (i.e., gastroscopy and colonoscopy), with or without biopsy, are not usually considered indications for antibiotic prophylaxis.[citation needed]
Notwithstanding the foregoing, the American Heart Association has recently changed its recommendations regarding antibiotic prophylaxis for endocarditis. Specifically, as of 2007, it is recommended that such prophylaxis be limited only to 1. those with prosthetic heart valves, 2. those with previous episode(s) of endocarditis, and 3. those with certain types of congenital heart disease.[9][Dead Link]
Since the stenosed aortic valve may limit the heart's output, people with aortic stenosis are at risk of syncope and dangerously low blood pressure should they use any of a number of medications for cardiovascular diseases that often co-exist with aortic stenosis. Examples include nitroglycerin, nitrates, ACE inhibitors, terazosin (Hytrin), and hydralazine. Note that all of these substances lead to peripheral vasodilation. Under normal circumstances, in the absence of aortic stenosis, the heart is able to increase its output and thereby offset the effect of the dilated blood vessels. In some cases of aortic stenosis, however, due to the obstruction of blood flow out of the heart caused by the stenosed aortic valve, cardiac output cannot be increased. Low blood pressure or syncope may ensue.
Neonatal
The above is entirely about adult aortic stenosis. In the new born, abnormalities of the left ventricle, the aortic valve or other heart and aortic abnomalities may lead the Ductus Ateriosus remaining open, or the Duct may remain open with no other abnormality. See Patent Ductus Arteriosus (PDA) Patent ductus arteriosus Then, and only then, in neonates, will the following advice be appropriate.
1.Stabilize with prostaglandin E1(PGE) infusion to maintain cardiac output through PDA. 2. Inotropic support as needed.
There is no medical treatment for aortic stenosis. Symptoms need assessment for surgery, severe symptoms may need urgent surgery. Meanwhile, dysrythmias or hypercholesterolaemia may need medical treatment.
Treatment
Treatment is generally not necessary in asymptomatic patients.[6] In moderate cases, echocardiography is performed every 1–2 years to monitor the progression, possibly complemented with a cardiac stress test.[3] In severe cases, echocardiography is performed every 3–6 months.[3] In both moderate and mild cases, the patient should immediately make a revisit or be admitted for inpatient care if any new related symptoms appear.[3]
Aortic valve replacement
Main article: aortic valve replacementIn adults, symptomatic aortic stenosis usually requires aortic valve replacement (AVR). AVR has been the standard of care for aortic stenosis for several decades.
Apicoaortic Conduit
Apicoaortic Conduit (AAC), or Aortic Valve Bypass (AVB), has been shown to be an effective treatment for aortic stenosis.[10] There is long-term stability of the left ventricular hemodynamics after AVB, with no further biologic progression of native aortic valve stenosis. Once the pressure gradient across the native valve is substantially reduced, the narrowing and calcification of the native valve halts.
Percutaneous Aortic Valve Replacement
According to a prospective, single-center, nonrandomized study of 25 patients, percutaneous implantation of an aortic valve prosthesis in high-risk patients with aortic stenosis results in marked hemodynamic and clinical improvement when successfully completed.[11] Medium- and long-term results are unknown. When selecting the optimal therapy for individual patients, the percutaneous approach must be carefully weighed against the excellent results achieved with conventional surgery.
Balloon valvuloplasty
For infants and children, balloon valvuloplasty, where a balloon is inflated to stretch the valve and allow greater flow, may also be effective. In adults, however, it is generally ineffective, as the valve tends to return to a stenosed state. The surgeon will make a small incision at the top of the patient's leg and proceed to insert the balloon into the artery and then inflate it to get a better flow of blood around the patient's body.[12]
Medical
In general, any medical therapy has relatively poor effect in treating aortic stenosis.[6] It is useful, however, in management of concomitant conditions that correlate with aortic stenosis:
- Any angina is generally treated with beta-blockers and/or calcium blockers[3]. Nitrates are contraindicated due to their potential to cause profound hypotension in aortic stenosis. [13]
- Any hypertension is treated aggressively, but caution must be taken in administering beta-blockers[3]
- Any heart failure is generally treated with digoxin and diuretics, and, if not contraindicated, cautious inpatient administration of ACE inhibitors[3]. As for angina, nitrates are contraindicated.
Since calcific aortic stenosis shares many pathological features and risk factors with atherosclerosis, and since atherosclerosis may be prevented and/or reversed by cholesterol lowering, there has been interest in attempting to modify the course of calcific aortic stenosis by cholesterol lowering with statin drugs. Although a number of small, observational studies demonstrated an association between lowered cholesterol and decreased progression, and even regression, of calcific aortic stenosis, a recent, large randomized clinical trial, published in 2005, failed to find any predictable effect of cholesterol lowering on calcific aortic stenosis. A 2007 study did demonstrate a slowing of aortic stenosis with the statin rosuvastatin.[14] However, what is likely to be considered the definitive trial, published in the New England Journal of Medicine in 2008, failed to find any beneficial effect of intensive cholesterol lowering on the course of aortic stenosis.[15]
See also
References
- ^ Aortic stenosis at Mount Sinai Hospital
- ^ Lilly LS (editor) (2003). Pathophysiology of Heart Disease (3rd ed.). Lippincott Williams & Wilkins. ISBN 0-7817-4027-4.
- ^ a b c d e f g h i j k l m n VOC=VITIUM ORGANICUM CORDIS, a compendium of the Department of Cardiology at Uppsala Academic Hospital. By Per Kvidal September 1999, with revision by Erik Björklund May 2008
- ^ Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997; 29: 630-634.
- ^ clinical Anesthesiology by Edward Morgan
- ^ a b c d e f g h i j k l m Chapter 1: Diseases of the Cardiovascular system > Section: Valvular Heart Disease in: Elizabeth D Agabegi; Agabegi, Steven S. (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-7153-6.
- ^ a b Etchells E, Bell C, Robb K (February 1997). "Does this patient have an abnormal systolic murmur?". JAMA 277 (7): 564–71. doi:10.1001/jama.277.7.564. PMID 9032164.
- ^ Lilly, editor, Leonard S. (2007). Pathophysiology of heart disease : a collaborative project of medical students and faculty (4th ed. ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. pp. 36. ISBN 978-0-7-8176321-9.
- ^ Infective Endocarditis (previously referred to as bacterial endocarditis)
- ^ Vliek CJ, Balaras E, Li S, Lin JY, Young CA, DeFillippi CR, Griffith BP, Gammie JS, Early and Midterm Hemodynamics After Aortic Valve Bypass (Apicoaortic Conduit) Surgery, Ann Thorac Surg 2010;90:136–43.
- ^ Grube E, Laborde JC, Gerckens U et al. (2006). "Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study". Circulation 114 (15): 1616–24. doi:10.1161/CIRCULATIONAHA.106.639450. PMID 17015786.
- ^ Mayo Clinic > Aortic valve stenosis > Treatments and drugs Retrieved September 2010
- ^ 1 Rutherford SD, Braunwald E. Chronic ischaemic heart disease. In: Braunwald E, ed. Heart disease: A textbook of cardiovascular medicine. 4th ed. Philadelphia: WB Saunders, 1992:1292-1364.
- ^ Moura LM, Ramos SF, Zamorano JL et al. (2007). "Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis". J. Am. Coll. Cardiol. 49 (5): 554–61. doi:10.1016/j.jacc.2006.07.072. PMID 17276178.
- ^ Rossebø AB, Pedersen TR, Boman K et al. (September 2008). "Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis". N. Engl. J. Med. 359 (13): 1343–56. doi:10.1056/NEJMoa0804602. PMID 18765433. http://content.nejm.org/cgi/content/abstract/359/13/1343.
External links
- Aortic valve stenosis clinical trials at ClinicalTrials.gov
- Aortic Stenosis Trial a clinical trial for Patients with Aortic Stenosis
- Teaching video: How to evaluate a patient with low-gradient aortic stenosis using echocardiography
- Study on the Effects of Critical Aortic Stenosis
Congenital heart defects (Q20–Q24, 745–746) Cardiac shunt/
heart septal defectAortopulmonary septal defectR→L: Double outlet right ventricle (Taussig–Bing syndrome) · Transposition of the great vessels (dextro, levo) · Persistent truncus arteriosusL→R: Sinus venosus atrial septal defect · Lutembacher's syndromeL→R and R→L: Eisenmenger's syndromeR→L, with other conditions: Tetralogy of FallotL→R: Ostium primumValvular heart disease/
heart chambersRightpulmonary valves (stenosis, insufficiency) · tricuspid valves (stenosis, atresia, Ebstein's anomaly) · Hypoplastic right heart syndrome (Uhl anomaly)Leftaortic valves (stenosis, insufficiency, bicuspid) · mitral valves (stenosis, regurgitation) · Hypoplastic left heart syndromeOther Dextrocardia · Levocardia · Cor triatriatum · Crisscross heart · Brugada syndrome · Coronary artery anomaly · Anomalous aortic origin of a coronary artery · Ventricular inversionCategories:- Valvular heart disease
- Diseases of the aorta
Wikimedia Foundation. 2010.