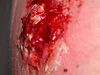
- Methicillin-resistant Staphylococcus aureus
-
"MRSA" redirects here; you may be looking for Metrolina Regional Scholars' Academy
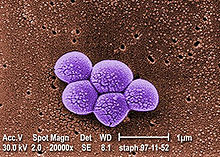
Methicillin-resistant Staphylococcus aureus 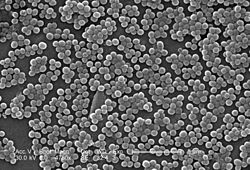
SEM micrograph of MRSA Scientific classification Kingdom: Bacteria Phylum: Firmicutes Class: Cocci Order: Bacillales Family: Staphylococcaceae Genus: Staphylococcus Species: S. aureus Binomial name Staphylococcus aureus
Rosenbach 1884Methicillin-resistant Staphylococcus aureus (MRSA) is a bacterium responsible for several difficult-to-treat infections in humans. It is also called multidrug-resistant Staphylococcus aureus and oxacillin-resistant Staphylococcus aureus (ORSA). MRSA is any strain of Staphylococcus aureus that has developed resistance to beta-lactam antibiotics, which include the penicillins (methicillin, dicloxacillin, nafcillin, oxacillin, etc.) and the cephalosporins. The development of such resistance does not cause the organism to be more intrinsically virulent than strains of Staphylococcus aureus that have no antibiotic resistance, but resistance does make MRSA infection more difficult to treat with standard types of antibiotics and thus more dangerous.
MRSA is especially troublesome in hospitals and nursing homes, where patients with open wounds, invasive devices, and weakened immune systems are at greater risk of infection than the general public.
Contents
Signs and symptoms
S. aureus most commonly colonizes the anterior nares (the nostrils). The rest of the respiratory tract, open wounds, intravenous catheters, and the urinary tract are also potential sites for infection. Healthy individuals may carry MRSA asymptomatically for periods ranging from a few weeks to many years. Patients with compromised immune systems are at a significantly greater risk of symptomatic secondary infection.
In most patients, MRSA can be detected by swabbing the nostrils and isolating the bacteria found inside. Combined with extra sanitary measures for those in contact with infected patients, screening patients admitted to hospitals has been found to be effective in minimizing the spread of MRSA in hospitals in the United States,[1] Denmark, Finland, and the Netherlands.[2]
MRSA may progress substantially within 24–48 hours of initial topical symptoms. After 72 hours, MRSA can take hold in human tissues and eventually become resistant to treatment. The initial presentation of MRSA is small red bumps that resemble pimples, spider bites, or boils; they may be accompanied by fever and, occasionally, rashes. Within a few days, the bumps become larger and more painful; they eventually open into deep, pus-filled boils.[3] About 75 percent of community-associated (CA-) MRSA infections are localized to skin and soft tissue and usually can be treated effectively. But some CA-MRSA strains display enhanced virulence, spreading more rapidly and causing illness much more severe than traditional healthcare-associated (HA-) MRSA infections, and they can affect vital organs and lead to widespread infection (sepsis), toxic shock syndrome, and necrotizing ("flesh-eating") pneumonia. This is thought to be due to toxins carried by CA-MRSA strains, such as PVL and PSM, though PVL was recently found to not be a factor in a study by the National Institute of Allergy and Infectious Diseases (NIAID) at the NIH. It is not known why some healthy people develop CA-MRSA skin infections that are treatable while others infected with the same strain develop severe infections or die.[4]
The most common manifestations of CA-MRSA are skin infections, such as necrotizing fasciitis and pyomyositis (most commonly found in the tropics), necrotizing pneumonia, infective endocarditis (which affects the valves of the heart), and bone and joint infections.[5] CA-MRSA often results in abscess formation that requires incision and drainage. Before the spread of MRSA into the community, abscesses were not considered contagious, because it was assumed that infection required violation of skin integrity and the introduction of staphylococci from normal skin colonization. However, newly emerging CA-MRSA is transmissible (similar, but with very important differences) from Hospital-Associated MRSA. CA-MRSA is less likely than other forms of MRSA to cause cellulitis.
Risk factors
Some of the populations at risk:
- People with weak immune systems (people living with HIV/AIDS, people living with lupus, cancer patients, transplant recipients, severe asthmatics, etc.)
- Diabetics
- Intravenous drug users
- Users of quinolone antibiotics[6]
- Young children
- The elderly
- College students living in dormitories
- People staying or working in a health care facility for an extended period of time
- People who spend time in coastal waters where MRSA is present, such as some beaches in Florida and the west coast of the United States[7][8]
- People who spend time in confined spaces with other people, including prison inmates, military recruits in basic training,[9] and individuals who spend considerable time in changerooms or gyms.
Hospital patients
Many MRSA infections occur in hospitals and healthcare facilities, with a higher incidence rate in nursing homes or long-term care facilities. Rates of MRSA infection are also increased in hospitalised patients who are treated with quinolones. Healthcare provider-to-patient transfer is common, especially when healthcare providers move from patient to patient without performing necessary hand-washing techniques between patients.[6][10]
Prison inmates
In confined environments such as prisons, with continual admission of new members who may typically be in poor health and adopt poor hygiene practices, there have been a number of challenges reported first in the U.S. and then in Canada. The earliest reports were made by the CDC in state prisons. Subsequently reports of a massive rise in skin and soft tissue infections were reported by the CDC in the Los Angeles County Jail system in 2001, and this has continued. Pan et al. reported on the changing epidemiology of MRSA skin infection in the San Francisco County Jail, noting the MRSA accounted for more than 70% of S. aureus infection in the jail by 2002. Lowy and colleagues reported on frequent MRSA skin infections in New York State Prisons. Two reports on inmates in Maryland have demonstrated frequent colonization with MRSA.
In the news media hundreds of reports of MRSA outbreaks in prisons appeared between 2000 and 2008. For example, in February 2008, The Tulsa County Jail in the U.S. State of Oklahoma started treating an average of twelve Staphylococcus cases per month.[11] A report on skin and soft tissue infections in the Cook County Jail in Chicago in 2004–05 demonstrated that MRSA was the most common cause of these infections among cultured lesions and furthermore that few risk factors were more strongly associated with MRSA infections than infections caused by methicillin-susceptible S. aureus. In response to these and many other reports on MRSA infections among incarcerated and recently incarcerated persons, the Federal Bureau of Prisons has released guidelines for the management and control of the infections although few studies provide an evidence base for these guidelines.
People in contact with live food-producing animals
Cases of MRSA have increased in livestock animals. CC398 is a new clone of MRSA that has emerged in animals and is found in intensively reared production animals (primarily pigs, but also cattle and poultry), where it can be transmitted to humans. Although being dangerous to humans CC398 is often asymptomatic in food-producing animals.[12]
A 2011 study reported 47% of the meat and poultry sold in surveyed U.S. grocery stores was contaminated with S. aureus and, of those, 52%—or 24.4% of the total—were resistant to at least three classes of antibiotics. "Now we need to determine what this means in terms of risk to the consumer," said Dr. Keim, a co-author of the paper.[13]
Athletes
In the United States, there have been increasing numbers of reports of outbreaks of MRSA colonization and infection through skin contact in locker rooms and gyms, even among healthy populations. A study published in the New England Journal of Medicine[14] linked MRSA to the abrasions caused by artificial turf. Three studies by the Texas State Department of Health found that the infection rate among football players was 16 times the national average. In October 2006, a high school football player was temporarily paralyzed from MRSA-infected turf burns. His infection returned in January 2007 and required three surgeries to remove infected tissue, as well as three weeks of hospital stay.[15] MRSA has also been found in the public school systems throughout the country.[16]
Children
MRSA is also becoming a problem in pediatric settings,[17] including hospital nurseries.[18] A 2007 study found that 4.6% of patients in U.S. health care facilities were infected or colonized with MRSA.[19]
Diagnosis
Diagnostic microbiology laboratories and reference laboratories are key for identifying outbreaks of MRSA. New rapid techniques for the identification and characterization of MRSA have been developed. This notwithstanding, the bacterium generally must be cultured via blood, urine, sputum, or other body fluid cultures, and grown up in the lab in sufficient numbers to perform these confirmatory tests first, so there is no quick and easy method to diagnose a MRSA infection, therefore initial treatment is often based upon 'strong suspicion' by the treating physician, since any delay in treating this type of infection can have fatal consequences. These techniques include Real-time PCR and Quantitative PCR and are increasingly being employed in clinical laboratories for the rapid detection and identification of MRSA strains.[20][21]
Another common laboratory test is a rapid latex agglutination test that detects the PBP2a protein. PBP2a is a variant penicillin-binding protein that imparts the ability of S. aureus to be resistant to oxacillin.[22]
Strains
A defining characteristic of MRSA is its ability to thrive in the presence of penicillin-like antibiotics, which normally prevent bacterial growth by inhibiting synthesis of cell-wall material. MRSA contains a gene, mecA, which stops β-lactam antibiotics from inactivating the enzymes (transpeptidases) that are critical to cell wall synthesis.
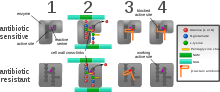 Diagram depicting antibiotic resistance through alteration of the antibiotic's target site, modeled after MRSA's resistance to penicillin. Beta-lactam antibiotics permanently inactivate PBP enzymes, which are essential for bacterial life, by permanently binding to their active sites. Some forms of MRSA, however, expresses a PBP that will not allow the antibiotic into its active site.
Diagram depicting antibiotic resistance through alteration of the antibiotic's target site, modeled after MRSA's resistance to penicillin. Beta-lactam antibiotics permanently inactivate PBP enzymes, which are essential for bacterial life, by permanently binding to their active sites. Some forms of MRSA, however, expresses a PBP that will not allow the antibiotic into its active site.
In the UK, where MRSA is commonly called "Golden Staph", the most common strains of MRSA are EMRSA15 and EMRSA16.[23] EMRSA16 is the best described epidemiologically: it originated in Kettering, England, and the full genomic sequence of this strain has been published.[24] EMRSA16 has been found to be identical to the ST36:USA200 strain, which circulates in the United States, and to carry the SCCmec type II, enterotoxin A and toxic shock syndrome toxin 1 genes.[25] Under the new international typing system, this strain is now called MRSA252. It is not entirely certain why this strain has become so successful, whereas previous strains have failed to persist. One explanation is the characteristic pattern of antibiotic susceptibility. Both the EMRSA15 and EMRSA16 strains are resistant to erythromycin and ciprofloxacin. It is known that Staphylococcus aureus can survive intracellularly,[26] for example in the nasal mucosa [27] and in the tonsil tissue ,.[28] Erythromycin and Ciprofloxacin are precisely the antibiotics that best penetrate intracellularly; it may be that these strains of S. aureus are therefore able to exploit an intracellular niche.
Community-acquired MRSA (CA-MRSA) is more easily treated, though more virulent, than hospital-acquired MRSA (HA-MRSA). CA-MRSA apparently did not evolve de novo in the community but represents a hybrid between MRSA that spread from the hospital environment and strains that were once easily treatable in the community. Most of the hybrid strains also acquired a factor that increases their virulence, resulting in the development of deep-tissue infections from minor scrapes and cuts, as well as many cases of fatal pneumonia.[29]
In the United States, most cases of CA-MRSA are caused by a CC8 strain designated ST8:USA300, which carries SCCmec type IV, Panton-Valentine leukocidin, PSM-alpha and enterotoxins Q and K,[25] and ST1:USA400.[30] Other community-acquired strains of MRSA are ST8:USA500 and ST59:USA1000. In many nations of the world, MRSA strains with different predominant genetic background types have come to predominate among CA-MRSA strains; USA300 easily tops the list in the U. S. and is becoming more common in Canada after its first appearance there in 2004. For example, in Australia ST93 strains are common, while in continental Europe ST80 strains predominate (Tristan et al., Emerging Infectious Diseases, 2006). In Taiwan, ST59 strains, some of which are resistant to many non-beta-lactam antibiotics, have arisen as common causes of skin and soft tissue infections in the community. In a remote region of Alaska, unlike most of the continental U. S., USA300 was found rarely in a study of MRSA strains from outbreaks in 1996 and 2000 as well as in surveillance from 2004–06 (David et al., Emerg Infect Dis 2008).
In June 2011, the discovery of a new strain of MRSA was announced by two separate teams of researchers in the UK. Its genetic make-up was reportedly more similar to strains found in animals, and testing kits designed to detect MRSA were unable to identify it.[31]
Prevention
Screening programs
Patient screening upon hospital admission, with nasal cultures, prevents the cohabitation of MRSA carriers with non-carriers, and exposure to infected surfaces. The test used (whether a rapid molecular method or traditional culture) is not as important as the implementation of active screening.[32] In the United States and Canada, the Centers for Disease Control and Prevention issued guidelines on October 19, 2006, citing the need for additional research, but declined to recommend such screening.[33][34]
In some UK hospitals screening for MRSA is performed in every patient[35] and all NHS surgical patients, except for minor surgeries, are previously checked for MRSA.[36] There is no community screening in the UK however screening of individuals is offered by some private companies.[37]
In a US cohort of 1300 healthy children, 2.4% carried MRSA in their nose.[38]
Surface sanitizing
Alcohol has been proven to be an effective surface sanitizer against MRSA. Quaternary ammonium can be used in conjunction with alcohol to extend the longevity of the sanitizing action.[39] The prevention of nosocomial infections involves routine and terminal cleaning. Non-flammable Alcohol Vapor in Carbon Dioxide systems (NAV-CO2) do not corrode metals or plastics used in medical environments and do not contribute to antibacterial resistance.
In healthcare environments, MRSA can survive on surfaces and fabrics, including privacy curtains or garments worn by care providers. Complete surface sanitation is necessary to eliminate MRSA in areas where patients are recovering from invasive procedures. Testing patients for MRSA upon admission, isolating MRSA-positive patients, decolonization of MRSA-positive patients, and terminal cleaning of patients' rooms and all other clinical areas they occupy is the current best practice protocol for nosocomial MRSA.
Studies published from 2004-2007 reported hydrogen peroxide vapor could be used to decontaminate busy hospital rooms, despite taking significantly longer than traditional cleaning. One study noted rapid recontamination by MRSA following the hydrogen peroxide application[40][41][42][43][44]
Also tested, in 2006, was a new type of surface cleaner, incorporating accelerated hydrogen peroxide, which was pronounced "a potential candidate" for use against the targeted microorganisms.[45]
Hand washing
In September 2004, [46] after a successful pilot scheme to tackle MRSA, the UK National Health Service announced its Clean Your Hands campaign. Wards were required to ensure that alcohol-based hand rubs are placed near all beds so that staff can hand wash more regularly. It is thought that even if this cuts infection by no more than 1%, the plan will pay for itself many times over.[citation needed]
As with some other bacteria, MRSA is acquiring more resistance to some disinfectants and antiseptics. Although alcohol-based rubs remain somewhat effective, a more effective strategy is to wash hands with running water and an anti-microbial cleanser with persistent killing action, such as Chlorhexidine[47]
A June 2008 report[citation needed], centered on a survey by the Association for Professionals in Infection Control and Epidemiology, concluded that poor hygiene habits remain the principal barrier to significant reductions in the spread of MRSA.
Use of surgical respirator
The U.S. Food and Drug Administration (FDA) announced on 8 April 2011 that it had cleared a novel type of N95 Surgical Respirator, the SpectraShield 9500, that kills methicillin-resistant Staphylococcus aureus, Streptococcus pyogenes and Haemophilus influenzae. This mask is manufactured by Nexera Medical Ltd. of Richmond, British Columbia The mask blocks at least 95% of small particles in a standardized test. The FDA clearance also included evaluation by the National Institute of Occupational Safety and Health.[48]
Proper disposal of hospital gowns
Used paper hospital gowns are associated with MRSA hospital infections, which could be avoided by proper disposal.[49]
Isolation
Current US guidance does not require workers in the general workplace (excluding medical facilities) with MRSA infections to be routinely excluded from going to work.[50] Therefore, unless directed by a health care provider, exclusion from work should be reserved for those with wound drainage that cannot be covered and contained with a clean, dry bandage and for those who cannot maintain good hygiene practices.[50] Workers with active infections should be excluded from activities where skin-to-skin contact is likely to occur until their infections are healed. Health care workers should follow the Centers for Disease Control and Prevention's Guidelines for Infection Control in Health Care Personnel.[51]
To prevent the spread of staph or MRSA in the workplace, employers should ensure the availability of adequate facilities and supplies that encourage workers to practice good hygiene; that surface sanitizing in the workplace is followed; and that contaminated equipment are sanitized with Environmental Protection Agency (EPA)-registered disinfectants.[50]
Restricting antibiotic use
Glycopeptides, cephalosporins and in particular quinolones are associated with an increased risk of colonisation of MRSA. Reducing use of antibiotic classes that promote MRSA colonisation, especially fluoroquinolones, is recommended in current guidelines.[6][10]
Public health considerations
Mathematical models describe one way in which a loss of infection control can occur after measures for screening and isolation seem to be effective for years, as happened in the UK. In the "search and destroy" strategy that was employed by all UK hospitals until the mid-1990s, all patients with MRSA were immediately isolated, and all staff were screened for MRSA and were prevented from working until they had completed a course of eradication therapy that was proven to work. Loss of control occurs because colonised patients are discharged back into the community and then readmitted; when the number of colonised patients in the community reaches a certain threshold, the "search and destroy" strategy is overwhelmed.[52] One of the few countries not to have been overwhelmed by MRSA is the Netherlands: An important part of the success of the Dutch strategy may have been to attempt eradication of carriage upon discharge from hospital.[53]
The Centers for Disease Control and Prevention (CDC) estimated that about 1.7 million nosocomial infections occurred in the United States in 2002, with 99,000 associated deaths.[54] The estimated incidence is 4.5 nosocomial infections per 100 admissions, with direct costs (at 2004 prices) ranging from $10,500 (£5300, €8000 at 2006 rates) per case (for bloodstream, urinary tract, or respiratory infections in immunocompetent patients) to $111,000 (£57,000, €85,000) per case for antibiotic-resistant infections in the bloodstream in patients with transplants. With these numbers, conservative estimates of the total direct costs of nosocomial infections are above $17 billion. The reduction of such infections forms an important component of efforts to improve healthcare safety. (BMJ 2007)[citation needed] MRSA alone was associated with 8% of nosocomial infections reported to the CDC National Healthcare Safety Network from January 2006 to October 2007.[55]
This problem is not unique to one country; the British National Audit Office estimated that the incidence of nosocomial infections in Europe ranges from 4% to 10% of all hospital admissions. As of early 2005, the number of deaths in the United Kingdom attributed to MRSA has been estimated by various sources to lie in the area of 3,000 per year.[56] Staphylococcus bacteria account for almost half of all UK hospital infections. The issue of MRSA infections in hospitals has recently been a major political issue in the UK, playing a significant role in the debates over health policy in the United Kingdom general election held in 2005.
On January 6, 2008, half of 64 non-Chinese cases of MRSA infections in Hong Kong in 2007 were Filipino domestic helpers. Ho Pak-leung, professor of microbiology at the University of Hong Kong, traced the cause to high use of antibiotics. In 2007, there were 166 community cases in Hong Kong compared with 8,000 hospital-acquired MRSA case (155 recorded cases—91 involved Chinese locals, 33 Filipinos, 5 each for Americans and Indians, and 2 each from Nepal, Australia, Denmark and England).[57]
Worldwide, an estimated 2 billion people carry some form of S. aureus; of these, up to 53 million (2.7% of carriers) are thought to carry MRSA.[58] In the United States, 95 million carry S. aureus in their noses; of these, 2.5 million (2.6% of carriers) carry MRSA.[59] A population review conducted in three U.S. communities showed the annual incidence of CA-MRSA during 2001–2002 to be 18–25.7/100,000; most CA-MRSA isolates were associated with clinically relevant infections, and 23% of patients required hospitalization.[60]
One possible contribution to the increased spread of MRSA infections comes from the use of antibiotics in intensive pig farming. A 2008 study in Canada found MRSA in 10% of tested pork chops and ground pork; a U.S. study in the same year found MRSA in the noses of 70% of the tested farm pigs and in 45% of the tested pig farm workers.[61] There have also been anecdotal reports of increased MRSA infection rates in rural communities with pig farms.[62]
Healthcare facilities with high bed occupancy rates, high levels of temporary nursing staff, or low cleanliness scores no longer have significantly higher MRSA rates. Simple tabular evidence helps provide a clear picture of these changes, showing, for instance, that hospitals with occupancy over 90% had, in 2006–2007, MRSA rates little above those in hospitals with occupancy below 85%, in contrast to the period 2001–2004. In one sense, the disappearance of these relationships is puzzling. Reporters now blame IV cannula and catheters for spreading MRSA in hospitals. (Hospital organisation and speciality mix, 2008)[citation needed]
Decolonization
Care should be taken when trying to drain boils, as disruption of surrounding tissue can lead to larger infections, or even infection of the blood stream (often with fatal consequences).[63] Any drainage should be disposed of very carefully. After the drainage of boils or other treatment for MRSA, patients can shower at home using chlorhexidine (Hibiclens) or hexachlorophene (Phisohex) antiseptic soap (available over-the-counter at many pharmacies) from head to toe. Alternatively, a dilute bleach bath can be taken at a concentration of 1/2 cup bleach per 1/4-full bathtub of water.[64] Care should be taken to use a clean towel, and to ensure that nasal discharge (i.e. snot) doesn't infect the towel (see below).
All infectious lesions should be kept covered with a dressing (band-aids etc.).[63] Mupirocin (Bactroban) 2% ointment can be effective at reducing the size of lesions. A secondary covering of clothing is preferred.[65]
The nose is a common refuge for MRSA, however a test swab can be taken of the nose to indicate whether MRSA is present.[66] Mupirocin (Bactroban) 2% ointment can be applied inside each nostril twice daily for 7 days, using a cotton-tipped swab. However, care should be taken so that the swab doesn't penetrate into the sinus. Household members are recommended to follow the same decolonization protocol. After treatment, the nose should be swabbed again to ensure that the treatment was effective. If not, the process should be repeated.
Toilet seats are a common vector for infection, therefore the seat can be wiped clean before and/or after each use. Door handles, faucets, light switches (with care!), etc. can be disinfected daily (or regularly; use of disinfectant wipes is recommended).[65] Spray disinfectants can be used on upholstery. Carpets can be washed with disinfectant, and hardwood floors can be scrubbed with diluted tea tree oil (e.g. Melaleuca). Laundry soap containing tea tree oil may be effective at decontaminating clothing and bedding, especially if hot water and heavy soil cycles are used, however tea tree oil may cause a rash which MRSA can re-colonize. Alcohol-based sanitizers can be placed near bedsides, near sitting areas, in vehicles etc. to encourage their use.
Doctors may also prescribe antibiotics such as clindamycin, doxycycline or trimethoprim/sulfamethoxazole. Although very few studies have shown that using more antibiotics actually has the effect of preventing recurrent MRSA skin infections,[67] anecdotal evidence and common sense demonstrate that antibiotics reduce the size of any infections and therefore reduce the ability of MRSA to spread.
Treatment
Both CA-MRSA and HA-MRSA are resistant to traditional anti-staphylococcal beta-lactam antibiotics, such as cephalexin. CA-MRSA has a greater spectrum of antimicrobial susceptibility, including to sulfa drugs (like co-trimoxazole/trimethoprim-sulfamethoxazole), tetracyclines (like doxycycline and minocycline) and clindamycin, but the drug of choice for treating CA-MRSA has is now believed to be Vancomycin, according to a Henry Ford Hospital Study. The study was presented on October 23, 2010, at the 48th annual meeting of the Infectious Diseases Society of America in Vancouver. HA-MRSA is resistant even to these antibiotics and often is susceptible only to vancomycin. Newer drugs, such as linezolid (belonging to the newer oxazolidinones class) and daptomycin, are effective against both CA-MRSA and HA-MRSA.
Vancomycin and teicoplanin are glycopeptide antibiotics used to treat MRSA infections.[68] Teicoplanin is a structural congener of vancomycin that has a similar activity spectrum but a longer half-life.[69] Because the oral absorption of vancomycin and Teicoplanin is very low, these agents must be administered intravenously to control systemic infections.[70] Drugs are administered via a Peripherally inserted central catheter, or a Picc Line, which is inserted by radiologists, doctors, physician assistants (in the U.S.), radiologist assistants (in the U.S.), or specially trained certified registered nurses.[71] Treatment of MRSA infection with vancomycin can be complicated, due to its inconvenient route of administration. Moreover, many clinicians believe that the efficacy of vancomycin against MRSA is inferior to that of anti-staphylococcal beta-lactam antibiotics against methicillin-susceptible Staphylococcus aureus (MSSA).[72][73]
Several newly discovered strains of MRSA show antibiotic resistance even to vancomycin and teicoplanin. These new evolutions of the MRSA bacterium have been dubbed Vancomycin intermediate-resistant Staphylococcus aureus (VISA).[74] [75] Linezolid, quinupristin/dalfopristin(synercid), daptomycin, and tigecycline are used to treat more severe infections that do not respond to glycopeptides such as vancomycin.[76]
There have been claims that bacteriophage can be used to cure MRSA.[77] [78]
The psychedelic mushroom Psilocybe semilanceata has been shown to strongly inhibit the growth of Staphylococcus aureus.[79]
Initial studies at the University of East London have demonstrated that allicin (a compound found in garlic) exhibits a strong antimicrobial response to the bacteria, indicating that it may one day lead to more effective treatments.[80]
A report released in 2010 details the efficacy of the active ingredients of a new composite dressing (hydrogen peroxide, tobramycin, chlorhexidine digluconate, chlorhexidine gluconate, levofloxacin, and silver) against MRSA.[81]
A 1990 study tested MRSA isolates obtained from veterans and found they could be killed by several substances, including bacitracin, nitrofurantoin, hydrogen peroxide, novobiocin, netilmicin and vancomycin. The study went on to conclude that netilmicin might be useful as an alternative to intravenous vancomycin, and suggested that topical applications of hydrogen peroxide may be useful to reduce MRSA on skin and some mucous membranes.[82]
History
US and UK
MRSA was discovered in 1961 in the United Kingdom. It made its first major appearance in the United States in 1981 among intravenous drug users. MRSA is often referred to in the press as a "superbug". The number of MRSA infections in the United States has been increasing significantly. A 2007 report in Emerging Infectious Diseases, a publication of the Centers for Disease Control and Prevention (CDC), estimated the number of MRSA infections in hospitals doubled nationwide, from approximately 127,000 in 1999 to 278,000 in 2005, while at the same time annual deaths increased from 11,000 to more than 17,000.[83] Another study led by the CDC and published in the October 17, 2007 issue of the Journal of the American Medical Association estimated that MRSA was responsible for 94,360 serious infections and associated with 18,650 hospital stay-related deaths in the United States in 2005.[84][85] These figures suggest that MRSA infections are responsible for more deaths in the U.S. each year than AIDS.[86]
The Office for National Statistics reported 1,629 MRSA-related deaths in England and Wales during 2005, indicating a MRSA-related mortality rate half the rate of that in the United States for 2005, even though the figures from the British source were explained to be high because of "improved levels of reporting, possibly brought about by the continued high public profile of the disease"[87] during the time of the 2005 United Kingdom General Election. MRSA is thought to have caused 1,652 deaths in 2006 in UK up from 51 in 1993.[88]
It has been argued that the observed increased mortality among MRSA-infected patients may be the result of the increased underlying morbidity of these patients. Several studies, however, including one by Blot and colleagues, that have adjusted for underlying disease still found MRSA bacteremia to have a higher attributable mortality than methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia.[89]
While the statistics suggest a national epidemic growing out of control, it has been difficult to quantify the degree of morbidity and mortality attributable to MRSA. A population-based study of the incidence of MRSA infections in San Francisco during 2004–05 demonstrated that nearly 1 in 300 residents suffered from such an infection in the course of a year and that greater than 85% of these infections occurred outside of the healthcare setting.[90] A 2004 study showed that patients in the United States with S. aureus infection had, on average, three times the length of hospital stay (14.3 vs. 4.5 days), incurred three times the total cost ($48,824 vs $14,141), and experienced five times the risk of in-hospital death (11.2% vs 2.3%) than patients without this infection.[91] In a meta-analysis of 31 studies, Cosgrove et al.,[92] concluded that MRSA bacteremia is associated with increased mortality as compared with MSSA bacteremia (odds ratio = 1.93; 95% CI = 1.93±0.39).[93] In addition, Wyllie et al. report a death rate of 34% within 30 days among patients infected with MRSA, a rate similar to the death rate of 27% seen among MSSA-infected patients.[94]
MRSA is sometimes sub-categorised as community-acquired MRSA (CA-MRSA) or healthcare-associated MRSA (HA-MRSA), although the distinction is complex. Some researchers have defined CA-MRSA by the characteristics of patients whom it infects, while others define it by the genetic characteristics of the bacteria themselves.
Worldwide
The first reported cases of CA-MRSA began to appear in the mid-1990s in Australia, New Zealand, the United States, the United Kingdom, France, Finland, Canada and Samoa, and were notable because they involved people who had not been exposed to a healthcare setting.[5]
In 1997, four fatal cases were reported involving children from Minnesota and North Dakota.[5] Over the next several years, it became clear that CA-MRSA infections were caused by strains of MRSA that differed from the older and better studied health care-associated strains.[95]
Research
Clinical
It has been reported that maggot therapy to clean out necrotic tissue of MRSA infection has been successful. Studies in diabetic patients reported significantly shorter treatment times than those achieved with standard treatments.[96][97][98]
Many antibiotics against MRSA are in phase II and phase III clinical trials. e.g.:
- Phase III : ceftobiprole, Ceftaroline, Dalbavancin, Telavancin, Aurograb, torezolid, iclaprim...
- Phase II : nemonoxacin.[99]
Pre-clinical
An entirely different and promising approach is phage therapy (e.g., at the Eliava Institute in Georgia[100]), which in mice had a reported efficacy against up to 95% of tested Staphylococcus isolates.[101]
On May 18, 2006, a report in Nature identified a new antibiotic, called platensimycin, that had demonstrated successful use against MRSA.[102][103]
A 2010 study noted significant antimicrobial action of Ulmo 90 and manuka UMF 25+ honey against several microorganisms, including MRSA. The investigators noted the superior antimicrobial action of Ulmo 90 honey, and suggested it be investigated further.[104] A separate 2010 study examined the use of medical-grade honey against several antibiotic-resistant strains of bacteria, including MRSA. The study concluded that the antimicrobial action of the honey studied was due to the activity of hydrogen peroxide, methylglyoxal, and a novel compound named bee defensin-1.[105]
Ocean-dwelling living sponges produce compounds that may make MRSA more susceptible to antibiotics.[106]
Some semi-toxic fungi/mushrooms excrete broad spectrum antibiotics, not all of which have been fully identified.[107]
Cannabinoids (components of Cannabis sativa), including cannabidiol (CBD), cannabinol (CBN), cannabichromene (CBC) and cannabigerol (CBG), show activity against a variety of MRSA strains. [108]
See also
References
- ^ Study at the Veterans Affairs hospital in Pittsburgh: "Science Daily". http://www.sciencedaily.com/upi/index.php?feed=Science&article=UPI-1-20070727-15235200-bc-us-infections.xml.[dead link]
- ^ McCaughey B. "Unnecessary Deaths: The Human and Financial Costs of Hospital Infections" (PDF). Archived from the original on July 11, 2007. http://web.archive.org/web/20070711030535/http://www.tufts.edu/med/apua/Patients/ridbooklet.pdf. Retrieved 2007-08-05.
- ^ "Symptoms". Mayo Clinic. http://www.mayoclinic.com/health/mrsa/DS00735/DSECTION=symptoms.
- ^ "MRSA Toxin Acquitted: Study Clears Suspected Key to Severe Bacterial Illness". NIH news release. National Institute of Health. 2006-11-06. http://www3.niaid.nih.gov/news/newsreleases/2006/staphtoxin.htm.
- ^ a b c Raygada JL and Levine DP (March 30, 2009). "Managing CA-MRSA Infections: Current and Emerging Options". Infections in Medicine 26 (2). http://www.consultantlive.com/infection/article/1145625/1393856.
- ^ a b c Tacconelli, E.; De Angelis, G.; Cataldo, MA.; Pozzi, E.; Cauda, R. (Jan 2008). "Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis.". J Antimicrob Chemother 61 (1): 26–38. doi:10.1093/jac/dkm416. PMID 17986491. http://jac.oxfordjournals.org/cgi/content/full/61/1/26.
- ^ Reuters (2009-02-16). "Study: Beachgoers More Likely to Catch MRSA". FoxNews.com. http://www.foxnews.com/story/0,2933,493604,00.html.
- ^ Marilynn Marchione (2009-09-12). "Dangerous staph germs found at West Coast beaches". AP. http://www.foxnews.com/story/0,2933,549601,00.html.
- ^ Zinderman, C.; Conner, B.; Malakooti, M.; LaMar, J.; Armstrong, A.; Bohnker, A. (May 2004). "Community-Acquired Methicillin-Resistant Staphylococcus aureus Among Military Recruits". Emerging Infectious Diseases. http://www.medscape.com/viewarticle/474843.
- ^ a b Muto, CA.; Jernigan, JA.; Ostrowsky, BE.; Richet, HM.; Jarvis, WR.; Boyce, JM.; Farr, BM. (May 2003). "SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus". Infect Control Hosp Epidemiol 24 (5): 362–86. doi:10.1086/502213. PMID 12785411.
- ^ Staph (MRSA) Infection Eradicated For 14 Months
- ^ "Joint scientific report of ECDC, EFSA and EMEA on meticillin resistant Staphylococcus aureus (MRSA) in livestock, companion animals and food". 2009-06-16. http://www.efsa.europa.eu/EFSA/Report/biohaz_report_301_joint_mrsa_en,0.pdf. Retrieved 2009-09-19.
- ^ http://www.sciencedaily.com/releases/2011/04/110415083153.htm
- ^ "New England Journal of Medicine". http://content.nejm.org/cgi/content/full/352/5/468.
- ^ Epstein, Victor (21 December 2007). "Texas Football Succumbs to Virulent Staph Infection From Turf". Bloomberg. http://www.bloomberg.com/apps/news?pid=newsarchive&sid=alxhrJDn.cdc. Retrieved 10 June 2010.
- ^ "SVSD410". http://svsd410.org/districtinfo/newspubs/news.asp?DistrictNewsID=262.[dead link]
- ^ Gray JW (April 2004). "MRSA: the problem reaches paediatrics". Arch. Dis. Child. 89 (4): 297–8. doi:10.1136/adc.2003.045534. PMC 1719885. PMID 15033832. http://adc.bmjjournals.com/cgi/content/full/89/4/297.
- ^ Bratu S, Eramo A, Kopec R, et al. (June 2005). "Community-associated methicillin-resistant Staphylococcus aureus in hospital nursery and maternity units". Emerging Infect. Dis. 11 (6): 808–13. PMID 15963273. http://www.cdc.gov/ncidod/EID/vol11no06/04-0885.htm.
- ^ Association for Professionals in Infection Control & Epidemiology (June 25, 2007). "National Prevalence Study of Methicillin-Resistant Staphylococcus aureus (MRSA) in U.S. Healthcare Facilities". Archived from the original on September 7, 2007. http://web.archive.org/web/20070907201425/http://www.apic.org/Content/NavigationMenu/ResearchFoundation/NationalMRSAPrevalenceStudy/MRSA_Study_Results.htm. Retrieved 2007-07-14.
- ^ Francois P and Schrenzel J (2008). "Rapid Diagnosis and Typing of Staphylococcus aureus". Staphylococcus: Molecular Genetics. Caister Academic Press. ISBN 9781904455295. http://www.horizonpress.com/staph.
- ^ Mackay I M (editor). (2007). Real-Time PCR in Microbiology: From Diagnosis to Characterization. Caister Academic Press. ISBN 9781904455189. http://www.horizonpress.com/rtmic.
- ^ Seiken, Denka. "MRSA latex test for PBP2". http://www.hardydiagnostics.com/catalog2/hugo/MRSALatexTest.htm.
- ^ Johnson AP, Aucken HM, Cavendish S, et al. (2001). "Dominance of EMRSA-15 and -16 among MRSA causing nosocomial bacteraemia in the UK: analysis of isolates from the European Antimicrobial Resistance Surveillance System (EARSS)". J Antimicrob Chemother 48 (1): 143–4. doi:10.1093/jac/48.1.143. PMID 11418528. http://jac.oxfordjournals.org/cgi/content/full/48/1/143.
- ^ Holden MTG, Feil EJ, Lindsay JA, et al. (2004). "Complete genomes of two clinical Staphylococcus aureus strains: Evidence for the rapid evolution of virulence and drug resistance". Proc Natl Acad Sci USA 101 (26): 9786–91. doi:10.1073/pnas.0402521101. PMC 470752. PMID 15213324. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=470752.
- ^ a b Diep B, Carleton H, Chang R, Sensabaugh G, Perdreau-Remington F (2006). "Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus". J Infect Dis 193 (11): 1495–503. doi:10.1086/503777. PMID 16652276.
- ^ von Eiff C, Becker K, Metze D, et al. (2001). "Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease". Clin Infect Dis 32 (11): 1643–7. doi:10.1086/320519. PMID 11340539.
- ^ Clement S, Vaudaux P, François P, et al. (2005). "Evidence of an intracellular reservoir in the nasal mucosa of patients with recurrent Staphylococcus aureus rhinosinusitis". J Infect Dis 192 (6): 1023–8. doi:10.1086/432735. PMID 16107955.
- ^ Zautner AE, Krause M, Stropahl G, et al. (2010). Bereswill, Stefan. ed. "Intracellular persisting Staphylococcus aureus is the major pathogen in recurrent tonsillitis". PloS One 5 (3): e9452. doi:10.1371/journal.pone.0009452. PMC 2830486. PMID 20209109. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2830486.
- ^ "Community-Associated meticillin-resistant Staphylococcusaureus: an emerging threat" (PDF). The Lancet. http://coe.ed.uidaho.edu/uploads/9/documents/MRSA%20review%207-05.pdf.
- ^ Wang R, Braughton KR, Kretschmer D, et al. (December 2007). "Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA". Nat. Med. 13 (12): 1510–4. doi:10.1038/nm1656. PMID 17994102.
- ^ http://www.irishtimes.com/newspaper/frontpage/2011/0603/1224298323851.html
- ^ Tacconelli E, De Angelis G, de Waure C, et al. (2009). "Rapid screening tests for meticillin-resistant Staphylococcus aureus at hospital admission: systematic review and meta-analysis". Lancet Infect Dis 9 (9): 546–554. doi:10.1016/S1473-3099(09)70150-1.
- ^ "To Catch a Deadly Germ," New York Times opinion
- ^ CDC Guideline "Management of Multidrug-Resistant Organisms in Healthcare Settings, 2006"
- ^ http://www.halifaxcourier.co.uk/latest-york-and-humberside-news/New-checks-in-hospitals-to.5123093.jp
- ^ "MRSA test for surgical patients". BBC News. 2009-03-31. http://news.bbc.co.uk/1/hi/health/7974964.stm. Retrieved 2010-04-05.
- ^ http://www.mrsatest.co.uk
- ^ Fritz SA, Garbutt J, Elward A, et al. (2008). "Prevalence of and risk factors for community-acquired methicillin-resistant and methicillin-sensitive Staphylococcus aureus colonization in children seen in a practice-based research network". Pediatrics 121 (6): 1090–8. doi:10.1542/peds.2007-2104. PMID 18519477.
- ^ Angela L. Hollingsworth. "AOAC Use Dilution Test Health Care" (PDF). http://www.sanisys.com/pdf_epa_salmo.pdf. Retrieved 2003-09-26.
- ^ Otter JA, Puchowicz M, Ryan D, et al. (June 2009). "Feasibility of routinely using hydrogen peroxide vapor to decontaminate rooms in a busy United States hospital". Infect Control Hosp Epidemiol 30 (6): 574–7. doi:10.1086/597544. PMID 19415969. http://www.journals.uchicago.edu/doi/abs/10.1086/597544?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed.
- ^ Bartels MD, Kristoffersen K, Slotsbjerg T, Rohde SM, Lundgren B, Westh H (September 2008). "Environmental meticillin-resistant Staphylococcus aureus (MRSA) disinfection using dry-mist-generated hydrogen peroxide". J. Hosp. Infect. 70 (1): 35–41. doi:10.1016/j.jhin.2008.05.018. PMID 18621434. http://linkinghub.elsevier.com/retrieve/pii/S0195-6701(08)00216-8.
- ^ French GL, Otter JA, Shannon KP, Adams NM, Watling D, Parks MJ (May 2004). "Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination". J. Hosp. Infect. 57 (1): 31–7. doi:10.1016/j.jhin.2004.03.006. PMID 15142713. http://linkinghub.elsevier.com/retrieve/pii/S019567010400101X.
- ^ Otter JA, Cummins M, Ahmad F, van Tonder C, Drabu YJ (October 2007). "Assessing the biological efficacy and rate of recontamination following hydrogen peroxide vapour decontamination". J. Hosp. Infect. 67 (2): 182–8. doi:10.1016/j.jhin.2007.07.019. PMID 17884250. http://linkinghub.elsevier.com/retrieve/pii/S0195-6701(07)00255-1.
- ^ Hardy KJ, Gossain S, Henderson N, et al. (August 2007). "Rapid recontamination with MRSA of the environment of an intensive care unit after decontamination with hydrogen peroxide vapour". J. Hosp. Infect. 66 (4): 360–8. doi:10.1016/j.jhin.2007.05.009. PMID 17655975. http://linkinghub.elsevier.com/retrieve/pii/S0195-6701(07)00173-9.
- ^ Omidbakhsh N, Sattar SA (June 2006). "Broad-spectrum microbicidal activity, toxicologic assessment, and materials compatibility of a new generation of accelerated hydrogen peroxide-based environmental surface disinfectant". Am J Infect Control 34 (5): 251–7. doi:10.1016/j.ajic.2005.06.002. PMID 16765201. http://linkinghub.elsevier.com/retrieve/pii/S0196-6553(05)00575-4.
- ^ "NPSA - About us". http://www.npsa.nhs.uk/cleanyourhands/about-us/.
- ^ Demarco, E.; Cushing, A.; Frempong-Manso, E.; Seo, M.; Jaravaza, A.; Kaatz, W. (Sep 2007). "Efflux-Related Resistance to Norfloxacin, Dyes, and Biocides in Bloodstream Isolates of Staphylococcus aureus" (Free full text). Antimicrobial Agents and Chemotherapy 51 (9): 3235–3239. doi:10.1128/AAC.00430-07. ISSN 0066-4804. PMC 2043220. PMID 17576828. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=17576828.
- ^ Gever J.Senior Editor, MedPage Today. Germicidal Surgical Mask Approved Published: April 09, 2011 http://www.medpagetoday.com/HospitalBasedMedicine/InfectionControl/25819
- ^ "Simple techniques slash hospital infections: meeting". Reuters. 2009-03-21. http://www.reuters.com/article/healthNews/idUSTRE52K1O920090321.
- ^ a b c "NIOSH MRSA and the Workplace". United States National Institute for Occupational Safety and Health. http://www.cdc.gov/niosh/topics/mrsa/. Retrieved 2007-10-29.
- ^ CDC (1998). "Guidelines for Infection Control in Health Care Personnel, 1998". Centers for Disease Control and Prevention. http://www.cdc.gov/ncidod/dhqp/gl_hcpersonnel.html. Retrieved December 18, 2007.
- ^ Cooper BS, Medley GF, Stone SP, et al. (2004). "Methicillin-resistant Staphylococcus aureus in hospitals and the community: stealth dynamics and control catastrophes". Proceedings of the National Academy of Sciences 101 (27): 10223–8. doi:10.1073/pnas.0401324101. PMC 454191. PMID 15220470. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=454191.
- ^ Bootsma MC, Diekmann O, Bonten MJ (2006). "Controlling methicillin-resistant Staphylococcus aureus: quantifying the effects of interventions and rapid diagnostic testing". Proc Natl Acad Sci USA 103 (14): 5620–5. doi:10.1073/pnas.0510077103. PMC 1459403. PMID 16565219. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1459403.
- ^ Klevens RM, Edwards JR, Richards CL, et al. (2007). "Estimating health care-associated infections and deaths in U.S. hospitals, 2002". Public Health Rep 122 (2): 160–6. PMC 1820440. PMID 17357358. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1820440.
- ^ Hidron AI, Edwards JR, Patel J, et al. (November 2008). "NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007". Infect Control Hosp Epidemiol 29 (11): 996–1011. doi:10.1086/591861. PMID 18947320.
- ^ Johnson AP, Pearson A, Duckworth G (2005). "Surveillance and epidemiology of MRSA bacteraemia in the UK". J Antimicrob Chemother 56 (3): 455–62. doi:10.1093/jac/dki266. PMID 16046464.
- ^ Inquirer.net, Cases of RP maids with 'superbug' infection growing in HK
- ^ "MRSA Infections". Keep Kids Healthy. http://www.keepkidshealthy.com/welcome/infectionsguide/mrsa.html.
- ^ Graham P, Lin S, Larson E (2006). "A U.S. population-based survey of Staphylococcus aureus colonization". Ann Intern Med 144 (5): 318–25. PMID 16520472.
- ^ Jernigan JA, Arnold K, Heilpern K, Kainer M, Woods C, Hughes JM (2006-05-12). "Methicillin-resistant Staphylococcus aureus as community pathogen". Symposium on Community-Associated Methicillin-resistant Staphylococcus aureus (Atlanta, Georgia, U.S.). Cited in Emerg Infect Dis. Centers for Disease Control and Prevention. http://www.cdc.gov/ncidod/EID/vol12no11/06-0911.htm. Retrieved 2007-01-27.
- ^ First study finds MRSA in U.S. pigs and farmers, seattlepi.com, 4 June 2008
- ^ Our Pigs, Our Food, Our Health, The New York Times, 12 March 2009
- ^ a b "PubMed Health". US National Institutes of Health. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0004520/. Retrieved 20 November 2011.
- ^ "Optimal Bleach Concentration Required to Kill MRSA in Bath Water". American Academy of Pediatrics.
- ^ a b "Living With MRSA". Group Health Cooperative/Tacoma-Pierce County Health Dept./Washington State Dept. of Health. http://www.tpchd.org/files/library/2357adf2a147d1aa.pdf. Retrieved 20 November 2011.
- ^ "THE NOSE – “GROUND ZERO” FOR MRSA COLONIZATION". Ondine Biomedical Inc. http://mrsatopic.com/2011/01/the-nose-ground-zero-for-mrsa-colonization/. Retrieved 20 November 2011.
- ^ Buckingham, SC (December 2008). "Prevention of Recurrent MRSA Skin Infections: What You Need to Know". Consultant 48 (13). http://www.consultantlive.com/display/article/10162/1360561.
- ^ Schentag JJ, Hyatt JM, Carr JR, Paladino JA, Birmingham MC, Zimmer GS, Cumbo TJ (1998). "Genesis of methicillin-resistant Staphylococcus aureus (MRSA), how treatment of MRSA infections has selected for vancomycin-resistant Enterococcus faecium, and the importance of antibiotic management and infection control". Clin. Infect. Dis. 26 (5): 1204–14. doi:10.1086/520287. PMID 9597254.
- ^ Rybak MJ, Lerner SA, Levine DP, Albrecht LM, McNeil PL, Thompson GA, Kenny MT, Yuh L (1991). "Teicoplanin pharmacokinetics in intravenous drug abusers being treated for bacterial endocarditis". Antimicrob. Agents Chemother. 35 (4): 696–700. PMC 245081. PMID 1829880. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=245081.
- ^ Janknegt R (1997). "The treatment of staphylococcal infections with special reference to pharmacokinetic, pharmacodynamic, and pharmacoeconomic considerations". Pharmacy world & science : PWS 19 (3): 133–41. doi:10.1023/A:1008609718457. PMID 9259029.
- ^ Kirsten Edwards
- ^ Chang FY, Peacock JE Jr, Musher DM, et al. (2003). "Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study". Medicine (Baltimore) 82 (5): 333–9. doi:10.1097/01.md.0000091184.93122.09. PMID 14530782.
- ^ Siegman-Igra Y, Reich P, Orni-Wasserlauf R, Schwartz D, Giladi M. (2005). "The role of vancomycin in the persistence or recurrence of Staphylococcus aureus bacteraemia". Scand J Infect Dis 37 (8): 572–8. doi:10.1080/00365540510038488. PMID 16138425.
- ^ Sieradzki K, Tomasz A (1997). "Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus". J. Bacteriol. 179 (8): 2557–66. PMC 179004. PMID 9098053. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=179004.
- ^ Schito GC (2006). "The importance of the development of antibiotic resistance in Staphylococcus aureus". Clin Microbiol Infect 12 Suppl 1: 3–8. doi:10.1111/j.1469-0691.2006.01343.x. PMID 16445718.
- ^ Mongkolrattanothai K, Boyle S, Kahana MD, Daum RS (2003). "Severe Staphylococcus aureus infections caused by clonally related community-associated methicillin-susceptible and methicillin-resistant isolates". Clin. Infect. Dis. 37 (8): 1050–8. doi:10.1086/378277. PMID 14523769.
- ^ http://www.phageinternational.com/ptc.htm
- ^ http://www.wired.com/wired/archive/11.10/phages.html
- ^ Suay I, Arenal F, Asensio FJ, Basilio A, Cabello MA, Díez MT, García JB, González del Val A, Gorrochategui J, Hernández P, Peláez F, Vicente MF. (2000). "Screening of basidiomycetes for antimicrobial activities" (PDF). Antonie van Leeuwenhoek 78 (2): 129–39. doi:10.1023/A:1026552024021. PMID 11204765. http://www.springerlink.com/content/p65r2660651u7k76/fulltext.pdf.
- ^ Cutler R.R.; Wilson, P (2004). "Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureus". British journal of biomedical science 61 (2): 71–4. PMID 15250668.
- ^ Echague CG, Hair PS, Cunnion KM (September 2010). "A comparison of antibacterial activity against Methicillin-Resistant Staphylococcus aureus and gram-negative organisms for antimicrobial compounds in a unique composite wound dressing". Adv Skin Wound Care 23 (9): 406–13. doi:10.1097/01.ASW.0000383213.95911.bc. PMID 20729646. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=1527-7941&volume=23&issue=9&spage=406.
- ^ Flournoy DJ, Robinson MC (October 1990). "In vitro antimicrobial susceptibilities of 349 methicillin-resistant Staphylococcus aureus isolates from veterans". Methods Find Exp Clin Pharmacol 12 (8): 541–4. PMID 2093132.
- ^ Klein E, Smith DL, Laxminarayan R (2007). "Hospitalizations and Deaths Caused by Methicillin-Resistant Staphylococcus aureus, United States, 1999–2005". Emerg Infect Dis 13 (12): 1840–6. PMC 2876761. PMID 18258033. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2876761.
- ^ Klevens et al. (2007), "Invasive Methicillin-Resistant Staphylococcus aureus Infections in the United States". JAMA. Retrieved on 2007-10-31.
- ^ Centers for Disease Control and Prevention (October 17, 2007), "MRSA: Methicillin-resistant Staphylococcus aureus in Healthcare Settings
- ^ Stein R (October 17, 2007), "Drug-resistant staph germ's toll is higher than thought." Washington Post. Retrieved on 2007-10-19.
- ^ UK Office for National Statistics Online (February 22, 2007), "MRSA Deaths continue to rise in 2005"
- ^ Hospitals struck by new killer bug An article by Manchester free newspaper 'Metro', May 7, 2008
- ^ Blot S, Vandewoude K, Hoste E, Colardyn F (2002). "Outcome and attributable mortality in critically Ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus". Arch Intern Med 162 (19): 2229–35. doi:10.1001/archinte.162.19.2229. PMID 12390067.
- ^ Liu C, Graber CJ, Karr M, et al. (June 2008). "A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004–2005". Clin. Infect. Dis. 46 (11): 1637–46. doi:10.1086/587893. PMID 18433335. http://www.cid.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=18433335.
- ^ Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Smulders M, Lapetina E, Gemmen E (2005). "The Burden of Staphylococcus aureus Infections on Hospitals in the United States: An Analysis of the 2000 and 2001 Nationwide Inpatient Sample Database". Arch Intern Med 165 (15): 1756–1761. doi:10.1001/archinte.165.15.1756. PMID 16087824.
- ^ Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y (2005). "The impact of Methicillin Resistance in Staphylococcus aureus Bacteremia on Patient Outcomes: Mortality, Length of Stay, and Hospital Charges" (– Scholar search). Infection Control and Hospital Epidemiology 26 (2): 166–174. doi:10.1086/502522. PMID 15756888. http://www.journals.uchicago.edu/ICHE/journal/issues/v26n2/9885/9885.html.[dead link]
- ^ Hardy KJ, Hawkey PM, Gao F, Oppenheim BA (2004). "Methicillin resistant Staphylococcus aureus in the critically ill". British Journal of Anaesthesia 92 (1): 121–30. doi:10.1093/bja/aeh008. PMID 14665563.
- ^ Wyllie D, Crook D, Peto T (2006). "Mortality after Staphylococcus aureus bacteraemia in two hospitals in Oxfordshire, 1997–2003: cohort study". BMJ 333 (7562): 281. doi:10.1136/bmj.38834.421713.2F. PMC 1526943. PMID 16798756. http://bmj.bmjjournals.com/cgi/content/abstract/333/7562/281.
- ^ Okuma K, Iwakawa K, Turnidge J, et al. (2002). "Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community". J Clin Microbiol 40 (11): 4289–94. doi:10.1128/JCM.40.11.4289-4294.2002. PMC 139674. PMID 12409412. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139674.
- ^ Bowling FL, Salgami EV, Boulton AJ (2007). "Larval therapy: a novel treatment in eliminating methicillin-resistant Staphylococcus aureus from diabetic foot ulcers". Diabetes Care 30 (2): 370–1. doi:10.2337/dc06-2348. PMID 17259512.
- ^ "Maggots help cure MRSA patients". BBC News. 2007-05-02. http://news.bbc.co.uk/2/hi/uk_news/england/manchester/6614471.stm.
- ^ "Maggots rid patients of MRSA". EurekAlert!/AAAS. 2007-05-03. http://www.eurekalert.org/pub_releases/2007-05/uom-mrp050307.php.
- ^ ClinicalTrials.gov NCT00685698
- ^ Murphy, Clare (2007-08-13). "'Red Army' virus to combat MRSA". BBC News. http://news.bbc.co.uk/2/hi/health/6943779.stm.
- ^ Matsuzaki S, Yasuda M, Nishikawa H, Kuroda M, Ujihara T, Shuin T, Shen Y, Jin Z, Fujimoto S, Nasimuzzaman MD, Wakiguchi H, Sugihara S, Sugiura T, Koda S, Muraoka A, Imai S (2003). "Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage phi MR11". J. Infect. Dis. 187 (4): 613–24. doi:10.1086/374001. PMID 12599078.
- ^ Bayston R, Ashraf W, Smith T (2007). "Triclosan resistance in methicillin-resistant Staphylococcus aureus expressed as small colony variants: a novel mode of evasion of susceptibility to antiseptics". J. Antimicrob. Chemother. 59 (5): 848–53. doi:10.1093/jac/dkm031. PMID 17337510.
- ^ Wang J; Soisson, SM; Young, K; Shoop, W; Kodali, S; Galgoci, A; Painter, R; Parthasarathy, G et al. (May 2006). "Platensimycin is a selective FabF inhibitor with potent antibiotic properties". Nature 441 (7091): 358–361. doi:10.1038/nature04784. PMID 16710421.
- ^ Sherlock O, Dolan A, Athman R, et al. (2010). "Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa". BMC Complement Altern Med 10: 47. doi:10.1186/1472-6882-10-47. PMC 2942791. PMID 20813024. http://www.biomedcentral.com/1472-6882/10/47.
- ^ Kwakman PH, te Velde AA, de Boer L, Speijer D, Vandenbroucke-Grauls CM, Zaat SA (July 2010). "How honey kills bacteria". FASEB J. 24 (7): 2576–82. doi:10.1096/fj.09-150789. PMID 20228250. http://www.fasebj.org/cgi/pmidlookup?view=long&pmid=20228250.
- ^ Sponge's secret weapon restores antibiotics' power
- ^ Psilocybe_semilanceata#Ecology_and_habitat
- ^ Appendino G, Gibbons S, Giana A, Pagani A, Grassi G, Stavri M, Smith E, Rahman M (2008). "Antibacterial Cannabinoids from Cannabis sativa: A Structure-Activity Study". J. Nat. Prod. 71 (8): 1427–30. doi:10.1021/np8002673. PMID 18681481. http://pubs.acs.org/doi/pdf/10.1021/np8002673.
Categories:- Staphylococcaceae
- Bacterial diseases
Wikimedia Foundation. 2010.