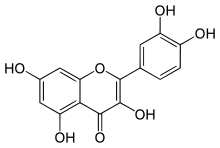
- Natural phenol
-
Natural phenols, bioavailable phenols, plant phenolics, low molecular weight phenols or phenoloids[1][2][3] are a class of natural products. They are small molecules containing one or more phenolic group. These molecules are smaller in size than polyphenols, containing less than 12 phenolic groups. They can be classified as simple phenols (monophenols), with only one phenolic group, or di- (bi-), tri- and oligophenols, with two, three or several phenolic groups respectively. They can be found in plants and have an antioxidant activity.[4] They are the most widely distributed class of plant secondary metabolites and several thousand different compounds have been identified.[5]
Contents
Names
As natural phenols are most often found in plants, authors use the genus or species name of the plant the particular molecule is found in to form its vernacular name. For example, the name of the anthocyanidin petunidin comes from Petunia or the name viniferin comes from Vitis vinifera.
Chemistry
Structural features
Simple and mid-molecular weight phenolic dimers and trimers phenol substructures have various further nomenclatures depending on the number of phenolic hydroxyl groups. A phenol, per se, is the term for a substructure with one phenolic hydroxyl group, catechol- and resorcinol-types (benzenediols) have two, and pyrogallol- and phloroglucinol-types (benzenetriols) have three. Natural phenols may have heteroatom substituents other than hydroxyl groups; as might be expected, ether and ester linkages are common, as are various carboxylic acid derivatives.




Phenol Pyrocatechol Pyrogallol Resorcinol Phloroglucinol Natural phenol compositions are normally limited to carbon, hydrogen and oxygen in undefined proportion. As a class, they do not contain nitrogen, element characteristic of amino-acids (see tyrosine and L-DOPA), catecholamine hormones or alkaloids.
Chemical properties
The majority of these compounds are solubles molecules but the smaller molecules can be volatiles.
UV visible spectrum of gallic acid. UV visible spectrum of quercetin.
UV visible spectrum of quercetin.
Natural phenols spectral data show a typical UV absorbance characteristic of benzene aromaticity at 270 nm. However, according to Woodward's rules, bathochromic shifts often happen suggesting the presence of delocalised π electrons arising from a conjugation between the benzene and vinyls groups.[6]
Many natural phenols present chirality within their molecule. An example of such molecules is catechin. Cavicularin is a unusual macrocycle because it was the first compound isolated from nature displaying optical activity due to the presence of planar chirality and axial chirality.
Natural phenols chemically interact with many other substances. Stacking, a chemical property of molecules with aromaticity, is seen occurring between phenolic molecules. When studied in mass spectrometry, phenols easily form adduct ions with halogens. They can also interact with the food matrices or with different forms of silica (mesoporous silica, fumed silica[7] or silica-based sol gels[8]).
The largest and best studied natural phenols are the flavonoids, which include several thousand compounds, among them the flavonols, flavones, flavan-3ol (catechins), flavanones, anthocyanidins and isoflavonoids.[9]
Base Unit: 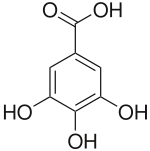
Gallic Acid 
Flavone 
Cinnamic acid Class/Polymer: Hydrolyzable tannins Flavonoid, Condensed tannins Lignins The phenolic unit can be found dimerized or further polymerized, creating a new class of polyphenol. For example, ellagic acid is a dimer of gallic acid and forms the class of ellagitannins, or a catechin and a gallocatechin can combine to form the red compound theaflavin, a process which also results in the large class of brown thearubigins in tea.
Two natural phenols from two different categories, for instance a flavonoid and a lignan, can combine to form a hybrid class like the flavonolignans.
Classification
There is a classification based on number of carbons based on Harborne et al., published in 1980:[10]
Number of carbon atoms Basic skeleton Number of phenolic cycles Class Examples 6 C6 1 Simple phenols, Benzoquinones Catechol, Hydroquinone, 2,6-Dimethoxybenzoquinone 7 C6-C1 1 Phenolic acids, Phenolic aldehydes Gallic, salicylic acids 8 C6-C2 1 Acetophenones, Tyrosine derivatives , Phenylacetic acids 3-Acetyl-6-methoxybenzaldehyde, Tyrosol, p-Hydroxyphenylacetic acid 9 C6-C3 1 Hydroxycinnamic acids, Phenylpropenes, Coumarins, Isocoumarins, Chromones Caffeic, ferulic acids, Myristicin, Eugenol, Umbelliferone, aesculetin, Bergenon, Eugenin 10 C6-C4 1 Naphthoquinones Juglone, Plumbagin 13 C6-C1-C6 2 Xanthonoids Mangiferin 14 C6-C2-C6 2 Stilbenoids, Anthraquinones Resveratrol, Emodin 15 C6-C3-C6 2 Chalconoids, Flavonoids, Isoflavonoids, Neoflavonoids Quercetin, cyanidin, Genistein 18 (C6-C3)2 2 Lignans, Neolignans Pinoresinol, Eusiderin 30 (C6-C3-C6)2 4 Biflavonoids Amentoflavone many (C6-C3)n,
(C6)n,
(C6-C3-C6)nn > 12 Lignins,
Catechol melanins,
Flavolans (Condensed tannins),
Polyphenolic proteins,
PolyphenolsRaspberry ellagitannin,
Tannic acidBiology
Ecology
Phenolic compounds are found in plants. They can also be found in mushroom basidiomycetes species. For example, protocatechuic acid and pyrocatechol are found in Agaricus bisporus[11] as well as other phenylated substances like phenylacetic and phenylpyruvic acids. The hardening of the protein component of insect cuticle has been shown to be due to the tanning action of an agent produced by oxidation of a phenolic substance. In the analogous hardening of the cockroach ootheca, the phenolic substance concerned is 3:4-dihydroxybenzoic acid (protocatechuic acid).[12]
Natural phenols can be involved in allelopathic interactions in soil.[13] Juglone is an example of such a molecule inhibiting the growth of other plant species around walnut trees. The nodule formation in Medicago truncatula is apparently dependent on the flavonoids pathway.[14] Pterocarpans serve as phytoalexins in Trifolium pratense and other Fabaceae.[15]
Furanocoumarins are phenolic and are non-toxic until activated by light. Furancoumarins block the transcription and repair of DNA. Therefore, they are considered phytotoxins.
Natural phenols can be enzymatically polymerised. Laccase and peroxidase induced the polymerization of syringic acid to give a poly(1,4-phenylene oxide) bearing a carboxylic acid at one end and a phenolic hydroxyl group at the other.[16]
In soils, it is assumed that larger amounts of phenols are released from decomposing plant litter rather than from throughfall in any natural plant community. In the soil, soluble phenols face four different fates. They might be degraded and mineralized as a carbon source by heterotrophic microorganisms; they can be transformed into insoluble and recalcitrant humic substances by polymerization and condensation reactions (with the contribution of soil organisms); they might adsorb to clay minerals or form chelates with aluminium or iron ions; or they might remain in dissolved form, leached by percolating water, and finally leave the ecosystem as part of dissolved organic carbon (DOC).[5]
Volatile phenolic compounds are found in plant resin where they may attract benefactors such as parasitoids or predators of the herbivores that attack the plant.[17]
Biological uses
Furanoflavonoids like karanjin or rotenoids are used as acaricide or insecticide.
Biosynthesis
Main article: Flavonoid biosynthesisMost of the natural phenols are derived from secondary plant metabolism of the shikimic acid pathway, malic acid pathway or both.[18] The aromatic amino acid phenylalanine, synthesized in the shikimic-acid pathway, is the common precursor of phenol containing amino acids and phenolic compounds.
In plants, the phenolic units are esterified or methylated. The polyphenols are submitted to conjugation. Many natural phenols are found in the glycoside form instead of the aglycone form.
In olive oil, tyrosol forms esters with fatty acids.[19] In rye, alkylresorcinols are phenolic lipids.
Some acetylations involve terpenes like geraniol.[20] Those molecules are called meroterpenes (a chemical compound having a partial terpenoid structure).
Methylations can occur by the formation of an ether bond on hydroxyl groups forming O-methylated polyphenols. In the case of the O-methylated flavone tangeritin, all of the five hydroxyls are methylated, leaving no free hydroxyls of the phenol group. Methylations can also occur on directly on a carbon of the benzene ring like in the case of poriol, a C-methylated flavonoid.
In animals and humans, after ingestion, polyphenols become part of the xenobiotic metabolism. In subsequent phase II reactions, these activated metabolites are conjugated with charged species such as glutathione, sulfate, glycine or glucuronic acid. These reactions are catalysed by a large group of broad-specificity transferases.
Content in human food
Main articles: Natural phenols and polyphenols in wine and Natural phenols and polyphenols in teaSee also: List of phytochemicals in foodNotable sources of natural phenols include berries, tea, beer, olive oil, chocolate or cocoa, coffee, pomegranates, popcorn, yerba mate, fruits and fruit based drinks (including cider and wine) and vegetables. Herbs and spices, nuts (walnuts, peanut) and algae are also potentially significant for supplying certain natural phenols. Such foods containing natural phenols are generally considered as health food.
Natural phenols can also be found in fatty matrices like olive oil.[21] Cloudy olive oil has the higher levels of phenols, or polar phenols that form a complex phenol-protein complex.
Phenolic compounds, when used in beverages, such as prune juice, have been shown to be helpful in the color and sensory components, such as alleviating bitterness.[22]
Health effects
Antioxidant activity
As interpreted by the Linus Pauling Institute and the European Food Safety Authority (EFSA), dietary flavonoids have little or no direct antioxidant food value following digestion.[23] Not like controlled test tube conditions, the fate of natural phenols in vivo shows they are poorly conserved (less than 5%), with most of what is absorbed existing as chemically-modified metabolites destined for rapid excretion.
Cinnamates have been shown to have more antioxidant activity when exposed in vitro to the Fenton reaction (catalytic Fe(II) with hydrogen peroxide) than the other natural phenols present in wine.[24]
In vitro effects
Mainly from in vitro studies, natural phenols have been reported to have antimicrobial,[25] antiviral,[26] anti-inflammatory,[27] and vasodilatory actions.[28]
Epigallocatechin gallate (EGCG), a flavanol found in tea, may have an effect on cancer by inhibiting DNA methyltransferase activity.[29] It has been shown to reduce reactive oxygen species levels in vitro.[30]
The natural phenol resveratrol inhibits occurrence and/or growth of experimental tumors.[31]
Research with biological model species
Natural phenols such as resveratrol activate human SIRT1, extend the lifespan of budding yeast, Saccharomyces cerevisiae,[32] and may be sirtuin-activating compounds.[33] Other examples of such products are butein, piceatannol, isoliquiritigenin, fisetin, and quercetin.[34] Longevity increased by resveratrol was demonstrated in Caenorhabditis elegans and Drosophila melanogaster.[35] This longevity increase may be due to a caloric restriction effect.[36] Resveratrol also increases the lifespan of vertebrates as has been demonstrated in short-lived fish, Nothobranchius furzeri.[37]
Other experiments on Drosophila melanogaster indicate that natural phenols (gallic acid, ferulic acid, caffeic acid, coumaric acid, propyl gallate, epicatechin, epigallocatechin, and epigallocatechin gallate) may influence mechanisms related to Parkinson's disease.[38] Quercetin and rutin act against scopolamine-induced memory impairment in zebrafish, Danio rerio.[39]
Potential risks
Neonatal carcinogenesis
Many natural phenols, like the flavonoids, were found to be strong topoisomerase inhibitors in vitro, some of them were tested in vivo with similar results[citation needed]. Those substances share the properity with some chemotherapeutic anticancer drugs such etoposide and doxorubicin[citation needed]. When tested some natural phenols induced DNA mutations in MLL gene, which are common findings in neonatal acute leukemia.[40] The DNA changes were highly increased by treatment with flavonoids in cultured blood stem cells.[41] Maternal high flavonoid content diet is suspected to increase risk of particularly acute myeloid leukemia in neonates.[42][43][44] Natural phenols have both anticarcinogenic - proapoptotic effect and a carcinogenic, DNA damaging, mutagenic potential. Adults seem to rapidly metabolize most of phenols, so toxic, mutagenic effect may not be pronounced in regular low doses intaken with food. Some natural phenols - ex. EGCG were found to rapidly induce detoxyfying Nrf2 transcription factor activity which seems to be responsible for observed beneficial, antioxidative effects of the substances and also leads to rapid degradation of the phenolic molecules. However human embryos detoxification system is not mature enough to deal with phenols, which have possibility to cross the placenta barier. High intake of flavonoid compounds during pregnancy is suspected to increase risk of neonatal leukemia.[40][43] Therefore "bioflavonoid" supplements should be not used by pregnant women.[45]
Other adverse effects
Phytoestrogens mainly belong to a large group of substituted natural phenolic compounds : the coumestans, prenylated flavonoids and isoflavones. Bisphenol A, a synthetic phenolic compound, is an endocrine disruptor, which can mimic the body's own hormones and may lead to negative health effects.
Research
Extraction can be performed using different solvents. There is a risk that polyphenol oxidase (PPO) degrades the phenolic content of the sample therefore there is a need to use PPO inhibitors like potassium dithionite (K2S2O4) or to perform experiment using liquid nitrogen or to boil the sample for a few seconds (blanching) to inactivate the enzyme.
pKa of phenolic compounds can be calculated from the retention time in liquid chromatography.[46][47]
Bioavailability
Questions on the relationship between health benefits and polyphenols generally revolve around bioavailability. Gallic acid and isoflavones are the most well-absorbed phenols, followed by catechins (flavan-3-ols), flavanones, and quercetin glucosides, but with different kinetics. The least well-absorbed phenols are the proanthocyanidins, galloylated tea catechins, and anthocyanins.[48]
Analytical methods
Studies on evaluating antioxidant capacity can used electrochemical methods.[49]
Detection can be made by recombinant luminescent bacterial sensors.[50]
Genetic analysis
The phenolic metabolic pahways and enzymes can be studied by mean of transgenesis of genes. The Arabidopsis regulatory gene Production of Anthocyanin Pigment 1 (AtPAP1) can be expressed in other plant species.[51]
See also
- Natural phenols and polyphenols in wine
- Natural phenols and polyphenols in tea
References
- ^ Detection of phenoloids in some Hungarian Inula and Centaurea species. A. Péter and G. Dósa, Acta Botanica Hungarica, Volume 44, Numbers 1-2 / March 2002, Pages 129-135, doi:10.1556/ABot.44.2002.1-2.9
- ^ LC-MS Analysis of Antioxidant Plant Phenoloids. I. Papp, P. Apáti, V. Andrasek, A. Blázovics, A. Balázs, L. Kursinszki, G. C. Kite, P. J. Houghton and Á. Kéry, Chromatographia, Volume 60, Supplement 1, S93-S100, doi:10.1365/s10337-004-0348-z
- ^ New chlorine-containing phenoloid from Curculigo capitulata. Ning Li, Tan Ning-Hua and Jun Zhou, Journal of Asian natural products research, 2004, vol. 6, no 1, pp. 7-10
- ^ Sources of natural phenolic antioxidants. Boskou Dimitrios, Trends in Food Science & Technology 17 (2006) 505–512, doi:10.1016/j.tifs.2006.04.00
- ^ a b The role of polyphenols in terrestrial ecosystem nutrient cycling. Stephan Hättenschwiler and Peter M. Vitouse, Trends in Ecology & Evolution, Volume 15, Issue 6, 1 June 2000, Pp. 238-243, doi:10.1016/S0169-5347(00)01861-9
- ^ Rapid analysis of stilbenes and derivatives from downy mildew-infected grapevine leaves by liquid chromatography–atmospheric pressure photoionisation mass spectrometry. J. Bernard Jean-Denis, Roger Pezet and Raffaele Tabacchi, Journal of Chromatography A, Volume 1112, Issues 1-2, 21 April 2006, Pages 263-268, doi:10.1016/j.chroma.2006.01.060
- ^ Interactions between bioactive ferulic acid and fumed silica by UV–vis spectroscopy, FT-IR, TPD MS investigation and quantum chemical methods. T.V. Kulik, N.A. Lipkovska, V.N. Barvinchenko, B.B. Palyanytsya, O.A. Kazakova, O.A. Dovbiy and V.K. Pogorelyi, Journal of Colloid and Interface Science, Volume 339, Issue 1, 1 November 2009, Pages 60-68, doi:10.1016/j.jcis.2009.07.055
- ^ Encapsulation of fluorescence vegetable extracts within a templated sol–gel matrix. Ioana Lacatusu, Nicoleta Badea, Rodica Nita, Alina Murariu, Florin Miculescu, Ion Iosub and Aurelia Meghea, Optical Materials, Volume 32, Issue 6, April 2010, Pages 711-718, 2nd International Conference on Functional Materials and Devices, 2nd International Conference on Functional Materials and Devices, doi:10.1016/j.optmat.2009.09.001
- ^ Jamison, Jennifer R.. Clinical Guide to Nutrition and Dietary Supplements in Disease Management. p. 525. ISBN 0-443-07193-4.
- ^ Harborne, J. B. (1980). "Plant phenolics". In Bell, E. A.; Charlwood, B. V.. Encyclopedia of Plant Physiology, volume 8 Secondary Plant Products. Berlin Heidelberg New York: Springer-Verlag. pp. 329–395.
- ^ Delsignore, A; Romeo, F; Giaccio, M (1997). "Content of phenolic substances in basidiomycetes". Mycological Research 101: 552–6. doi:10.1017/S0953756296003206.
- ^ The occurrence of phenolic substances in arthropods. R. H. Hackman, M. G. M. Pryor and A. R. Todd, Biochem J. 1948; 43(3): 474–477, PMC PMC1274717
- ^ Blum, Udo; Shafer, Steven R.; Lehman, Mary E. (1999). "Evidence for Inhibitory Allelopathic Interactions Involving Phenolic Acids in Field Soils: Concepts vs. an Experimental Model". Critical Reviews in Plant Sciences 18: 673–93. doi:10.1080/07352689991309441.
- ^ Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by Rhizobia. Anton P. Wasson, Flavia I. Pellerone and Ulrike Mathesius, The Plant Cell, Vol. 18, pp. 1617-1629, July 2006, doi:10.1105/tpc.105.038232
- ^ Biosynthesis of pterocarpan phytoalexins in Trifolium pratense. Paul M. Dewick, Phytochemistry, Volume 16, Issue 1, 1977, Pages 93-97, doi:10.1016/0031-9422(77)83020-3
- ^ Enzymatic Polymerization of Natural Phenol Derivatives and Enzymatic Synthesis of Polyesters from Vinyl Esters. Hiroshi Uyama, Ryohei Ikeda, Shigeru Yaguchi and Shiro Kobayashi, from book Polymers from Renewable Resources, Chapter 9, pp 113–127, ACS Symposium Series, Vol. 764, doi:10.1021/bk-2000-0764.ch009
- ^ "Plant Resins: Chemistry, evolution, ecology, and ethnobotany", by Jean Langenheim, Timber Press, Portland, OR. 2003
- ^ Knaggs, Andrew R. (2001). "The biosynthesis of shikimate metabolites (1999)". Natural Product Reports 18 (3): 334–55. doi:10.1039/b001717p. PMID 11476485.
- ^ Lucas, Ricardo; Comelles, Francisco; Alcántara, David; Maldonado, Olivia S.; Curcuroze, Melanie; Parra, Jose L.; Morales, Juan C. (2010). "Surface-Active Properties of Lipophilic Antioxidants Tyrosol and Hydroxytyrosol Fatty Acid Esters: A Potential Explanation for the Nonlinear Hypothesis of the Antioxidant Activity in Oil-in-Water Emulsions". Journal of Agricultural and Food Chemistry 58 (13): 8021–6. doi:10.1021/jf1009928. PMID 20524658.
- ^ Šmejkal, Karel; Grycová, Lenka; Marek, Radek; Lemière, Filip; Jankovská, Dagmar; Forejtníková, Hana; Vančo, Ján; Suchý, Václav (2007). "C-Geranyl Compounds from Paulownia tomentosa Fruits". Journal of Natural Products 70 (8): 1244–8. doi:10.1021/np070063w. PMID 17625893.
- ^ Gutfinger, T. (1981). "Polyphenols in olive oils". Journal of the American Oil Chemists Society 58: 966–8. doi:10.1007/BF02659771.
- ^ Phenolic Composition and Antioxidant Activity of Prunes and Prune Juice (Prunus domestica). Jennifer L. Donovan, Anne S. Meyer and Andrew L. Waterhouse, J. Agric. Food Chem. 1998, 46, pp. 1247−1252, doi:10.1021/jf970831x
- ^ Flavonoids: antioxidants or signalling molecules? Williams RJ, Spencer JP, Rice-Evans C, Free Radic Biol Med. 2004 Apr 1;36(7):838-49, doi:10.1016/j.freeradbiomed.2004.01.001 PMID 15019969
- ^ Novel Antioxidant Reactions of Cinnamates in Wine. Nick Emil Gislason, Bruce Lamonte Currie and Andrew Leo Waterhouse, J. Agric. Food Chem., 2011, 59 (11), pp 6221–6226, doi:10.1021/jf200115y
- ^ Rauha, J; Remes, S; Heinonen, M; Hopia, A; Kähkönen, M; Kujala, T; Pihlaja, K; Vuorela, H et al. (2000). "Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds". International Journal of Food Microbiology 56 (1): 3–12. doi:10.1016/S0168-1605(00)00218-X. PMID 10857921.
- ^ Perez, R.M. (2003). "Antiviral Activity of Compounds Isolated From Plants". Pharmaceutical Biology 41: 107–57. doi:10.1076/phbi.41.2.107.14240.
- ^ Dos Santos, Michel David; Almeida, Maria Camila; Lopes, Norberto Peporine; De Souza, Glória Emília Petto (2006). "Evaluation of the Anti-inflammatory, Analgesic and Antipyretic Activities of the Natural Polyphenol Chlorogenic Acid". Biological & Pharmaceutical Bulletin 29: 2236–40. doi:10.1248/bpb.29.2236.
- ^ Padilla, E; Ruiz, E; Redondo, S; Gordillo-Moscoso, A; Slowing, K; Tejerina, T (2005). "Relationship between vasodilation capacity and phenolic content of Spanish wines.". European journal of pharmacology 517 (1-2): 84–91. doi:10.1016/j.ejphar.2005.04.044. PMID 15967426.
- ^ Tea Polyphenol (−)-Epigallocatechin-3-Gallate Inhibits DNA Methyltransferase and Reactivates Methylation-Silenced Genes in Cancer Cell Lines. Ming Zhu Fang, Yimin Wang, Ni Ai, Zhe Hou, Yi Sun, Hong Lu, William Welsh and Chung S. Yang, Cancer Res November 15, 2003 63; 7563
- ^ Mei Y, Wei D, Liu J (April 2005). "Reversal of multidrug resistance in KB cells with tea polyphenol antioxidant capacity". Cancer Biol. Ther. 4 (4): 468–73. doi:10.4161/cbt.4.4.1698. PMID 15908794. http://www.landesbioscience.com/journals/cbt/abstract.php?id=1698.
- ^ Jang M, Cai L, Udeani GO, et al. (January 1997). "Cancer chemopreventive activity of resveratrol, a natural product derived from grapes". Science 275 (5297): 218–20. doi:10.1126/science.275.5297.218. PMID 8985016. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=8985016.
- ^ Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Konrad T. Howitz, Kevin J. Bitterman, Haim Y. Cohen, Dudley W. Lamming, Siva Lavu, Jason G. Wood, Robert E. Zipkin, Phuong Chung, Anne Kisielewski, Li-Li Zhang, Brandy Scherer and David A. Sinclair, Nature 425, 191-196 (11 September 2003), doi:10.1038/nature01960 PMID 12939617
- ^ Kaeberlein M, McDonagh T, Heltweg B, et al. (April 2005). "Substrate-specific activation of sirtuins by resveratrol". J. Biol. Chem. 280 (17): 17038–45. doi:10.1074/jbc.M500655200, PubMed
- ^ David A. Sinclair et al. Discover Three Classes of Molecules that Activate Sirtuins (Including Resveratrol)
- ^ Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Timothy M. Bass, David Weinkove, Koen Houthoofd, David Gems and Linda Partridge, Mechanisms of Ageing and Development, Volume 128, Issue 10, October 2007, Pages 546-552, doi:10.1016/j.mad.2007.07.007 PMID 17875315
- ^ Plant “Polyphenolic” Small Molecules Can Induce a Calorie Restriction-Mimetic Life-Span Extension by Activating Sirtuins: Will “Polyphenols” Someday Be Used as Chemotherapeutic Drugs in Western Medicine? Stéphane Quideau, ChemBioChem, Volume 5, Issue 4, pages 427–430, April 2, 2004, doi:10.1002/cbic.200300835
- ^ Life Extension in the Short-Lived Fish Nothobranchius furzeri. Alessandro Cellerino, Life-Span Extension, Aging Medicine, 2009, Part 5, 157-171, doi:10.1007/978-1-60327-507-1_10
- ^ The Effects of Polyphenols on Survival and Locomotor Activity in Drosophila melanogaster Exposed to Iron and Paraquat. M. Jimenez-Del-Rio, C. Guzman-Martinez and C. Velez-Pardo, Neurochemical Research, Volume 35, Number 2, 227-238, doi:10.1007/s11064-009-0046-1
- ^ Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. S.K. Richetti, M. Blank, c, K.M. Capiotti, A.L. Piato, M.R. Bogo, M.R. Vianna and C.D. Bonan, Behavioural Brain Research, Volume 217, Issue 1, 2 February 2011, Pages 10-15, doi:10.1016/j.bbr.2010.09.027
- ^ a b Strick, R. (2000). "From the Cover: Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia". Proceedings of the National Academy of Sciences 97: 4790–5. doi:10.1073/pnas.070061297. PMC 18311. PMID 10758153. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=18311.
- ^ Van Waalwijk Van Doorn-Khosrovani, S. B.; Janssen, J.; Maas, L. M.; Godschalk, R. W.L.; Nijhuis, J. G.; Van Schooten, F. J. (2007). "Dietary flavonoids induce MLL translocations in primary human CD34+ cells". Carcinogenesis 28 (8): 1703–9. doi:10.1093/carcin/bgm102. PMID 17468513.
- ^ Ross, JA (1998). "Maternal diet and infant leukemia: a role for DNA topoisomerase II inhibitors?". International journal of cancer. Supplement 11: 26–8. PMID 9876473.
- ^ a b Ross, J. A. (2000). "Dietary flavonoids and the MLL gene: A pathway to infant leukemia?". Proceedings of the National Academy of Sciences 97: 4411–3. doi:10.1073/pnas.97.9.4411. PMC 34309. PMID 10781030. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=34309.
- ^ Spector, L. G. (2005). "Maternal Diet and Infant Leukemia: The DNA Topoisomerase II Inhibitor Hypothesis: A Report from the Children's Oncology Group". Cancer Epidemiology Biomarkers & Prevention 14: 651–5. doi:10.1158/1055-9965.EPI-04-0602. PMID 15767345.
- ^ Paolini, M (2003). "Avoidance of bioflavonoid supplements during pregnancy: a pathway to infant leukemia?". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 527: 99–101. doi:10.1016/S0027-5107(03)00057-5.
- ^ Prediction of pKa values of phenolic and nitrogen-containing compounds by computational chemical analysis compared to those measured by liquid chromatography. T. Hanai, K. Koizumi, T. Kinoshita, R. Arora and F. Ahmed, Journal of Chromatography A, Volume 762, Issues 1-2, 21 February 1997, Pages 55-61, 20th International Symposium on High Performance Liquid Phase Separation and Related Techniques, doi:10.1016/S0021-9673(96)01009-6
- ^ Spectrophotometric, potentiometric and chromatographic pKa values of polyphenolic acids in water and acetonitrile–water media. J.L. Beltrán, N. Sanli, G. Fonrodona, D. Barrón, G. Özkan and J. Barbosa, Analytica Chimica Acta 484 (2003) 253–264, doi:10.1016/S0003-2670(03)00334-9
- ^ Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Claudine Manach, Gary Williamson, Christine Morand, Augustin Scalbert and Christian Rémésy, American Journal of Clinical Nutrition, Vol. 81, No. 1, 230S-242S, January 2005, Dietary polyphenols and health: Proceedings of the 1st international conference on polyphenols and health
- ^ How Do Phenolic Compounds React toward Superoxide Ion? A Simple Electrochemical Method for Evaluating Antioxidant Capacity. Alice Ren, Marie-Laurence Abasq, Didier Hauchard and Philippe Hapiot, Anal. Chem., 2010, 82 (20), pp 8703–8710, doi:10.1021/ac101854w
- ^ Analysis of bioavailable phenols from natural samples by recombinant luminescent bacterial sensors. Anu Leedjärv, Angela Ivask, Marko Virta and Anne Kahru, Chemosphere, Volume 64, Issue 11, September 2006, Pages 1910-1919, doi:10.1016/j.chemosphere.2006.01.026
- ^ Purple Canola: Arabidopsis PAP1 Increases Antioxidants and Phenolics in Brassica napus Leaves. Xiang Li, Ming-Jun Gao, Hong-Yu Pan, De-Jun Cui and Margaret Y. Gruber, J. Agric. Food Chem., 2010, 58 (3), pp 1639–1645, doi:10.1021/jf903527y
External links
Databases
- phenol-explorer.eu a database dedicated to phenolics found in food by Augustin Scalbert, INRA Clermont-Ferrand, Unité de Nutrition Humaine (Human food unit)
- FlavonoidViewer.jp (Japanese, English), a database on flavonoids by Arita Group (Univ of Tokyo, RIKEN Plant Science Center, and Keio Univ), Nishioka Group (Kyoto and Keio Univ) and Kanaya Group (NAIST)
- ChEMBLdb, a database of bioactive drug-like small molecules by the European Bioinformatics Institute
See also: General Alkaloids Natural phenols Monoterpenoids Diterpenoids Antibiotics ←Enzyme cofactors Phytochemicals Misc: biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCategories:- Natural phenols
Wikimedia Foundation. 2010.