- Methyl iodide
-
Methyl iodide 
 IodomethaneOther namesMonoiodomethane, Methyl iodine, MeI, Halon 10001, UN 2644
IodomethaneOther namesMonoiodomethane, Methyl iodine, MeI, Halon 10001, UN 2644Identifiers CAS number 74-88-4 
PubChem 6328 ChemSpider 6088 
EC number 200-819-5 KEGG C18448 
ChEBI CHEBI:39282 
ChEMBL CHEMBL115849 
RTECS number PA9450000 Jmol-3D images Image 1 - CI
Properties Molecular formula CH3I Molar mass 141.94 g/mol Appearance Clear colourless liquid with acrid odor Density 2.28 g/cm3 (20 °C)[1] Melting point -66.45 °C, 207 K, -88 °F
Boiling point 42.43 °C, 316 K, 108 °F
Solubility in water 1.4 g/100 mL (20 °C) log P 1.51 Vapor pressure 50 kPa at 20 °C
53.32 at 25.3 °C
166.1 kPa at 55 °CRefractive index (nD) 1.531 Hazards MSDS MSDS at Oxford University EU classification Toxic (T), Carc. Cat. 3 R-phrases R21 R23/25 R37/38 R40 S-phrases (S1/2) S36/37 S38 S45 NFPA 704 Explosive limits 8.5 - 66% Threshold Limit Value 2 ppm (TWA), 5 ppm (STEL) LD50 0.78 mmol/kg (mouse, s.c.)[1]  iodide (verify) (what is:
iodide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Methyl iodide, also called iodomethane, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted by rice plantations in small amounts.[2] It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans and in lesser amounts on land due to terrestrial fungi and bacteria. Methyl iodide is used in organic synthesis as a source of methyl groups, and was approved for use as a pesticide by the United States Environmental Protection Agency in 2007.[3] Iodomethane is a pre-plant biocide used to control insects, plant parasitic nematodes, soil borne pathogens, and weed seeds. The compound is registered for use as a preplant soil treatment for field grown strawberries, peppers, tomatoes, stone fruits, tree nuts, grape vines, ornamentals and turf and nursery grown strawberries, stone fruits, tree nuts, and conifer trees.
Contents
Preparation and handling
Methyl iodide is formed via the exothermic reaction that occurs when iodine is added to a mixture of methanol with red phosphorus.[4] The iodinating reagent is phosphorus triiodide that is formed in situ:
Alternatively, it is prepared from the reaction of dimethyl sulfate with potassium iodide in the presence of calcium carbonate:[4]
- (CH3O)2SO2 + KI → CH3I + CH3OSO2OK
Methyl iodide can also be prepared by the reaction of methanol with potassium iodide, catalyzed by acid:
- CH3OH + KI + H2SO4 → CH3I + K2SO4 + H2O
The reaction is carried out at low temperature and the water generated in the reaction is trapped by excess sulfuric acid so the reaction is not reversible. The generated methyl iodide can be distilled from the reaction mixture.
Storage and purification
Like many organoiodide compounds, methyl iodide is typically stored in dark bottles to inhibit degradation cause by light to give iodine, giving degraded samples a purplish tinge. Commercial samples may be stabilized by copper or silver wire.[5] It can be purified by distillation followed by washing with Na2S2O3 to remove iodine.
Reactions
Methylation reagent
Methyl iodide is an excellent substrate for SN2 substitution reactions. It is sterically open for attack by nucleophiles, and iodide is a good leaving group. It is used for alkylating carbon, oxygen, sulfur, nitrogen, and phosphorus nucleophiles.[5] Unfortunately, it has a high equivalent weight: one mole of methyl iodide weighs almost three times as much as one mole of methyl chloride. On the other hand, methyl chloride and methyl bromide are gaseous, thus harder to handle; they are also weaker alkylating agents.
Iodides are generally expensive relative to the more common chlorides and bromides, though methyl iodide is reasonably affordable; on a commercial scale, the more toxic dimethyl sulfate is preferred, since it is both cheap and liquid. The iodide leaving group in methyl iodide may cause side reactions, as it is a powerful nucleophile. Finally, being highly reactive, methyl iodide is more dangerous for laboratory workers than related chlorides and bromides.
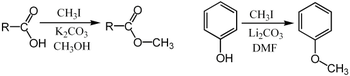
For example, it can be used for the methylation of carboxylic acids or phenols:[6]
In these examples, the base (K2CO3 or Li2CO3) removes the acidic proton to form the carboxylate or phenoxide anion, which serves as the nucleophile in the SN2 substitution.
Iodide is a "soft" anion which means that methylation with MeI tends to occur at the "softer" end of an ambidentate nucleophile. For example, reaction with thiocyanate ion favours attack at S rather than "hard" N, leading mainly to methyl thiocyanate (CH3SCN) rather than CH3NCS. This behavior is relevant to the methylation of stabilized enolates such as those derived from 1,3-dicarbonyl compounds. Methylation of these and related enolates can occur on the harder oxygen atom or the (usually desired) carbon atom. With methyl iodide, C-alkylation nearly always predominates.
Other reactions
In the Monsanto process, MeI forms in situ from the reaction of methanol and hydrogen iodide. The CH3I then reacts with carbon monoxide in the presence of a rhodium complex to form acetyl iodide, the precursor to acetic acid after hydrolysis. Most acetic acid is prepared by this method.
MeI is used to prepare the Grignard reagent, methylmagnesium iodide ("MeMgI"), a common source of "Me−. The use of MeMgI has been somewhat superseded by the commercially available methyllithium. MeI can also be used to prepare dimethylmercury, by reacting 2 moles of MeI with a 2/1-molar sodium amalgam (2 moles of sodium, 1 mol of mercury).
Use as a pesticide
Methyl iodide has also been proposed for use as a fungicide, herbicide, insecticide, nematicide, and as a soil disinfectant, replacing methyl bromide (also known as bromomethane) (banned under the Montreal Protocol). Manufactured by Arysta LifeScience and sold under the brand name MIDAS, methyl iodide is registered as a pesticide in the U.S., Mexico, Morocco, Japan, Turkey, and New Zealand and registration is pending in Australia, Guatemala, Costa Rica, Chile, Egypt, Israel, South Africa and other countries.[7] In September 2007, 54 chemists and physicians contacted the EPA expressing deep concerns of serious risk if methyl iodide were to be permitted for use in agriculture. In a letter to the EPA they stated: "We are skeptical of U.S. EPA’s conclusion that the high levels of exposure to methyl iodide that are likely to result from broadcast applications are “acceptable” risks. U.S. EPA has made many assumptions about toxicology and exposure in the risk assessment that have not been examined by independent scientific peer reviewers for adequacy or accuracy. Additionally, none of U.S. EPA’s calculations account for the extra vulnerability of the unborn fetus and children to toxic insults."[8] EPA Assistant Administrator Jim Gulliford replied saying, "We are confident that by conducting such a rigorous analysis and developing highly restrictive provisions governing its use, there will be no risks of concern," and in October the EPA approved the use of methyl iodide as a soil fumigant in the U.S. It could not, however, yet be used in Washington and New York due to lack of state approval. It also was not yet approved for use in California pending approval by the California growers Department of Pesticide Regulation. The California state agency often imposes tighter restrictions than the EPA, and the previous year its top officials had expressed concerns to the EPA about methyl iodide. "We are conducting our own risk assessment of methyl iodide, and we expect that process to continue for several months before we make a decision whether or how it can be used safely in California," said Glenn Brank, a spokesman for the state Department of Pesticide Regulation.[9][10]
In February 2010, the California Department of Pesticide Regulation (DPR) concluded that methyl iodide is "highly toxic," and that "any anticipated scenario for the agricultural or structural fumigation use of this agent would result in exposures to a large number of the public and thus would have a significant adverse impact on the public health." It also concluded that adequate control of the chemical in these circumstances would be "difficult, if not impossible."[11]
On December 1, 2010, methyl iodide use was approved as a pesticide in the State of California.[12] Following the approval, a coalition of environmentalists, researchers and farmers—including California State Assemblyman Bill Monning, D-Carmel—gathered at locations in Santa Cruz County and six other sites around California to protest the impending use of methyl iodide and to ask governor-elect Jerry Brown to block it. Critics of methyl iodide cite both the cancer-causing aspects of the pesticide and the potential for it to leach out of fields, contaminating water supplies.[13] Objections were also raised by two dozen California legislators and 54 scientists, including five Nobel laureates.[3]
On January 5, 2011, a coalition of farmworkers, community advocates and environmental health organizations sued to challenge California's approval of methyl iodide. The plaintiff groups are: Pesticide Action Network North America, United Farm Workers of America, Californians for Pesticide Reform, Pesticide Watch Education Fund, Worksafe, Communities and Children Advocates Against Pesticide Poisoning as well as farmworkers Jose Hidalgo Ramon and Zeferino Estrada. The suit challenges the state Department of Pesticide Regulation's December 20, 2010 approval of methyl iodide for use in California on the grounds that it violates the California Environmental Quality Act, the California Birth Defects Prevention Act, and the Pesticide Contamination Prevention Act. The suit also contends that the Department of Pesticide Regulation violated the law requiring involvement of the Office of Environmental Health Hazard Assessment in the development of farmworker safety regulations and made an unlawful finding of emergency with its request for Restricted Materials status for methyl iodide.[14]
The first commercial applications of MIDAS soil fumigant in California began in Fresno County, in May, 2011. To date since its federal registration as a pesticide, more than 17,000 acres of soil used for growing crops have been treated in the United States without a single reported safety or health incident. Soils treated with methyl iodide are completely free of the compound before crops are planted, and to date neither the U.S. EPA nor the U.S. Food and Drug Administration (agencies that routinely conduct pesticide residue testing of fruit and produce) have found any detectable quantity of methyl iodide in the food supply from fruit and produce grown in methyl iodide-treated soils, nor have they detected any other soil fumigant product.
Safety
Toxicity and biological effects
According to the United States Department of Agriculture methyl iodide exhibits moderate to high acute toxicity for inhalation and ingestion.[15] The Centers for Disease Control and Prevention (CDC) lists inhalation, skin absorption, ingestion, and eye contact as possible exposure routes with target organs of the eyes, skin, respiratory system, and the central nervous system. Symptoms may include eye irritation, nausea, vomiting, dizziness, ataxia, slurred speech, and dermatitis.[16]
Methyl iodide has an LD50 for oral administration to rats 76 mg/kg, and in the liver it undergoes rapid conversion to S-methylglutathione.[17]
In its risk assessment of methyl iodide, the U.S. EPA conducted an exhaustive scientific and medical literature search over the past 100 years for reported cases of human poisonings attributable to the compound. Citing the EPA as its source, the California Department of Pesticide Regulation concluded, “Over the past century, only 11 incidents of iodomethane poisoning have been reported in the published literature.” (Hermouet, C. et al 1996 & Appel, G.B. et al 1975) “An updated literature search on May 30, 2007 for iodomethane poisoning produced only one additional case report.” (Schwartz MD, et al 2005). All but one were industrial—not agricultural—accidents, and the remaining case of poisoning was an apparent suicide. Methyl iodide is routinely and regularly used in industrial processes as well as in most university and college chemistry departments for study and learning related to a variety of organic chemical reactions.
Carcinogenicity in mammals
Methyl iodide is listed under California Proposition 65 (1986) as a chemical known by the state to cause cancer or reproductive toxicity based on evaluative studies performed in the 1970s.[18] It is considered a potential occupational carcinogen by the U.S. National Institute for Occupational Safety and Health (NIOSH), the U.S. Occupational Safety and Health Administration and the U.S. Centers for Disease Control and Prevention.[19] The International Agency for Research on Cancer concluded based on studies performed after methyl iodide was Proposition 65 listed that: “Methyl iodide is not classifiable as to its carcinogenicity to humans (Group 3).” As of 2007[update] the Environmental Protection Agency classifies it as "not likely to be carcinogenic to humans in the absence of altered thyroid hormone homeostatis," i.e. it is a human carcinogen but only at doses large enough to disrupt thyroid function (via excess iodide).[20] However this finding is disputed by the Pesticide Action Network which states that the EPA’s cancer rating "appears to be based solely on a single rat inhalation study in which 66% of the control group and 54-62% of the rats in the other groups died before the end of the study". They go on to state: "The EPA appears to be dismissing early peer-reviewed studies in favor of two nonpeer-reviewed studies conducted by the registrant that are flawed in design and execution."[21] Despite requests by the U.S. EPA to the Pesticide Action Network to bring forth scientific evidence of their claims, they have not done so.
References
- ^ a b Merck Index, 11th Edition, 6002
- ^ K. R. Redeker, N.-Y. Wang, J. C. Low, A. McMillan, S. C. Tyler, and R. J. Cicerone (2000). "Emissions of Methyl Halides and Methane from Rice Paddies". Science 290 (5493): 966–969. doi:10.1126/science.290.5493.966. PMID 11062125.
- ^ a b Zitto, Kelly "Methyl iodide gains state OK for use on crops". San Francisco Chronicle. December 2, 2010. http://www.sfgate.com/cgi-bin/article.cgi?f=/c/a/2010/12/01/BAOQ1GKKKN.DTL.
- ^ a b King, C. S.; Hartman, W. W. (1943), "Methyl Iodide", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2P0399; Coll. Vol. 2: 399
- ^ a b Sulikowski, Gary A.; Sulikowski, Michelle M.; Haukaas, Michael H.; Moon, Bongjin (2005). "Iodomethane". e-EROS. doi:10.1002/047084289X.ri029m.pub2.
- ^ Avila-Zárraga, J. G., Martínez, R. (January 2001). "Efficient methylation of carboxylic acids with potassium hydroxide/methyl sulfoxide and iodomethane". Synthetic Communications 31 (14): 2177–2183. doi:10.1081/SCC-100104469.
- ^ "Iodomethane Approved in Mexico and Morocco". Business Wire. October 25, 2010. http://finance.yahoo.com/news/Iodomethane-Approved-in-bw-422120383.html?x=0.
- ^ http://www.wired.com/wiredscience/2007/10/scientists-stop/
- ^ "EPA approves new pesticide despite scientists' concerns". Los Angeles Times. October 6, 2007. http://www.latimes.com/news/printedition/california/la-me-pesticide6oct06,0,3454295.story.
- ^ "California sun and spray". High Country News. August 4, 2009. http://www.hcn.org/blogs/goat/california-sun-and-spray.
- ^ "Report of the Scientific Review Committee on Methyl Iodide to the Department of Pesticide Regulation". special Scientific Review Committee of the California Department of Pesticide Regulation. February 5, 2010. http://www.cdpr.ca.gov/docs/risk/mei/peer_review_report.pdf.
- ^ "Calif approves use of pesticide linked to cancer". San Francisco Chronicle. December 1, 2010. http://www.sfgate.com/cgi-bin/article.cgi?f=/n/a/2010/12/01/national/a143424S98.DTL&tsp=1.
- ^ "Statewide protest targets new strawberry pesticide". San Jose Mercury News. November 30, 2010. http://www.mercurynews.com/breaking-news/ci_16745459?nclick_check=1.
- ^ http://www.ens-newswire.com/ens/jan2011/2011-01-05-091.html
- ^ http://ddr.nal.usda.gov/bitstream/10113/28473/1/IND44184498.pdf
- ^ http://www.cdc.gov/niosh/npg/npgd0420.html
- ^ Johnson, M. K. (1966). "Metabolism of iodomethane in the rat". Biochem. J. 98 (1): 38–43. PMC 1264791. PMID 5938661. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1264791.
- ^ "Proposition 65: A Chemical Listed Effective April 15, 2011 As Known To The State Of California To Cause Cancer: Epoxiconazole (15 Apr 2011)". http://www.oehha.ca.gov/prop65/prop65_list/Newlist.html.
- ^ "CIB 43: MONOHALOMETHANES". http://www.cdc.gov/niosh/84117_43.html.
- ^ "Iodomethane Pesticide Fact Sheet". 2007. http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2005-0252-0056. (36 pages, inc 12 pages of refs)
- ^ http://www.cdpr.ca.gov/docs/risk/mei/comments/panna_mei_attach1.pdf
Additional sources
- March, Jerry (1992), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (4th ed.), New York: Wiley, ISBN 0-471-60180-2
- Sulikowski, G. A.; Sulikowski, M. M. (1999). in Coates, R.M.; Denmark, S. E. (Eds.) Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation New York: Wiley, pp. 423–26.
- Bolt H. M., Gansewendt B. (1993). "Mechanisms of carcinogenicity of methyl halides.". Crit Rev Toxicol. 23 (3): 237–53. doi:10.3109/10408449309105011. PMID 8260067.
External links
- International Chemical Safety Card 0509
- NIOSH Pocket Guide to Chemical Hazards 0420
- IARC Summaries & Evaluations: Vol. 15 (1977), Vol. 41 (1986), Vol. 71 (1999)
- Metabolism of iodomethane in the rat
- Iodomethane NMR spectra
- Jones, Nicola (September 24, 2009). "Strawberry pesticide leaves sour taste: Methyl iodide use by Californian farmers up for review.". Nature News. doi:10.1038/news.2009.943. http://www.nature.com/news/2009/090924/full/news.2009.943.html. Retrieved September 25, 2009.
Halomethanes Monosubstituted Disubstituted Trisubstituted Categories:- Organoiodides
- Halomethanes
- Methylating agents
- IARC Group 3 carcinogens
- Environmental controversies
- Environmental effects of pesticides
- Pesticides in the United States
Wikimedia Foundation. 2010.