- Dps (DNA-binding proteins from starved cells)
-
Dps (DNA-binding proteins from starved cells) 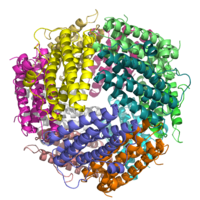
Structure of the DPS protein.[1] Identifiers Symbol DPS Entrez 1129452 PDB 1QGH UniProt P80725 Other data Dps (DNA-binding proteins from starved cells) proteins belong to the ferritin superfamily and are characterized by strong similarities but also distinctive differences with respect to “canonical” ferritins.
Dps proteins are part of a complex bacterial defence system that protects DNA against oxidative damage and are distributed widely in the bacterial kingdom.
Contents
Description
DPS are highly symmetrical dodecameric proteins of 200 kDa characterized from a shell-like structure of 2:3 tetrahedral symmetry assembled from identical subunits with an external diameter of ~ 9 nm and a central cavity of ~ 4.5 nm in diameter.[2][3][4] Dps proteins belong to the ferritin superfamily and the DNA protection is afforded by means of a double mechanism:
1) The first was discovered in Escherichia coli Dps in 1992 [5] and has given the name to the protein family; during stationary phase, Dps binds the chromosome non-specifically, forming a highly ordered and stable dps-DNA co-crystal within which chromosomal DNA is condensed and protected from diverse damages.[6] The lysine-rich N-terminus is required for self-aggregation as well as for Dps-driven DNA condensation.[7]
2) The second mode of protection is due to the ability of Dps proteins to bind and oxidize Fe(II) at the characteristic, highly conserved intersubunit ferroxidase center.[8][9]
The dinuclear ferroxidase centers are located at the interfaces between subunits related by 2-fold symmetry axes.[10] Fe(II) is sequestered and stored in the form of Fe(III) oxyhydroxide mineral, which can be released after reduction. In the mineral iron core up to 500 Fe(III) can be deposited. One hydrogen peroxide oxidizes two Fe2+ ions, which prevents hydroxyl radical production by the Fenton reaction (reaction I):
2 Fe2+ + H2O2 + 2 H+ = 2 Fe3+ + 2 H2O
Dps also protects the cell from UV and gamma irradiation, iron and copper toxicity, thermal stress and acid and base shocks.[11] Also shows a weak catalase activity.
Expression
In Escherichia coli Dps protein is Induced by rpoS and IHF in the early stationary phase. Dps is also Induced by oxyR in response to oxidative stress during exponential phase. ClpXP probably directly regulate proteolysis of dps during exponential phase. ClpAP seems to play an indirect role in maintaining ongoing dps synthesis during stationary phase
Applications
Cavities formed by Dps and ferritin proteins have been successfully used as the reaction chamber for the fabrication of metal nanoparticles (NPs).[12][13][14][15] Protein shells served as a template to restrain particle growth and as a coating to prevent coagulation/aggregation between NPs. Using various sizes of protein shells, various sizes of NPs can be easily synthesized for chemical, physical and bio-medical applications.
See also
References
- ^ Ilari, A.; Stefanini, S.; Chiancone, E.; Tsernoglou, D. (2000). "The dodecameric ferritin from Listeria innocua contains a novel intersubunit iron-binding site.". Nature structural biology 7 (1): 38–43. doi:10.1038/71236. PMID 10625425. Ilari, A.; Stefanini, S.; Chiancone, E.; Tsernoglou, D. (2000). "The dodecameric ferritin from Listeria innocua contains a novel intersubunit iron-binding site.". Nature structural biology 7 (1): 38–43. doi:10.1038/71236. PMID 10625425.
- ^ Grant, R. A.; Filman, D. J.; Finkel, S. E.; Kolter, R.; Hogle, J. M. (1998). "The crystal structure of Dps, a ferritin homolog that binds and protects DNA". Nature Structural Biology 5 (4): 294. doi:10.1038/nsb0498-294. PMID 9546221. Grant, RA; Filman, DJ; Finkel, SE; Kolter, R; Hogle, JM (1998). "The crystal structure of Dps, a ferritin homolog that binds and protects DNA". Nature structural biology 5 (4): 294–303. doi:10.1038/nsb0498-294. PMID 9546221.
- ^ Chiancone, E.; Ceci, P. (2010). "The multifaceted capacity of Dps proteins to combat bacterial stress conditions: Detoxification of iron and hydrogen peroxide and DNA binding". Biochimica et Biophysica Acta (BBA) - General Subjects 1800 (8): 798–805. doi:10.1016/j.bbagen.2010.01.013. PMID 20138126. Chiancone, E.; Ceci, P. (2010). "The multifaceted capacity of Dps proteins to combat bacterial stress conditions: Detoxification of iron and hydrogen peroxide and DNA binding". Biochimica et Biophysica Acta (BBA) - General Subjects 1800 (8): 798–805. doi:10.1016/j.bbagen.2010.01.013. PMID 20138126.
- ^ Chiancone, E.; Ceci, P. (2010). "Role of Dps (DNA-binding proteins from starved cells) aggregation on DNA". Frontiers in Bioscience 15 (1): 122. doi:10.2741/3610. PMID 20036810. Chiancone, E.; Ceci, P. (2010). "Role of Dps (DNA-binding proteins from starved cells) aggregation on DNA". Frontiers in bioscience : a journal and virtual library 15: 122–131. doi:10.2741/3610. PMID 20036810.
- ^ Almiron, M.; Link, A. J.; Furlong, D.; Kolter, R. (1992). "A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli". Genes & Development 6 (12B): 2646–54. doi:10.1101/gad.6.12b.2646. PMID 1340475. Almirón, M; Link, AJ; Furlong, D; Kolter, R (1992). "A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli". Genes & development 6 (12B): 2646–54. doi:10.1101/gad.6.12b.2646. PMID 1340475.
- ^ Wolf, S.; Frenkiel, D.; Arad, T.; Finkel, S.; Kolter, R.; Minsky, A. (1999). "DNA protection by stress-induced biocrystallization". Nature 400 (6739): 83–85. Bibcode 1999Natur.400...83W. doi:10.1038/21918. PMID 10403254. Wolf, S.; Frenkiel, D.; Arad, T.; Finkel, S.; Kolter, R.; Minsky, A. (1999). "DNA protection by stress-induced biocrystallization". Nature 400 (6739): 83–85. Bibcode 1999Natur.400...83W. doi:10.1038/21918. PMID 10403254.
- ^ Ceci, P.; Cellai, S.; Falvo, E.; Rivetti, C.; Rossi, G. L.; Chiancone, E. (2004). "DNA condensation and self-aggregation of Escherichia coli Dps are coupled phenomena related to the properties of the N-terminus". Nucleic Acids Research 32 (19): 5935. doi:10.1093/nar/gkh915. PMC 528800. PMID 15534364. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=528800. Ceci, P.; Cellai, S.; Falvo, E.; Rivetti, C.; Rossi, G. L.; Chiancone, E. (2004). "DNA condensation and self-aggregation of Escherichia coli Dps are coupled phenomena related to the properties of the N-terminus". Nucleic Acids Research 32 (19): 5935. doi:10.1093/nar/gkh915. PMC 528800. PMID 15534364. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=528800.
- ^ Zhao, G.; Ceci, P.; Ilari, A.; Giangiacomo, L.; Laue, T.; Chiancone, E.; Chasteen, N. (2002). "Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells. A ferritin-like DNA-binding protein of Escherichia coli". The Journal of biological chemistry 277 (31): 27689–27696. doi:10.1074/jbc.M202094200. PMID 12016214. Zhao, G.; Ceci, P.; Ilari, A.; Giangiacomo, L.; Laue, T.; Chiancone, E.; Chasteen, N. (2002). "Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells. A ferritin-like DNA-binding protein of Escherichia coli". The Journal of biological chemistry 277 (31): 27689–27696. doi:10.1074/jbc.M202094200. PMID 12016214.
- ^ Ceci, P.; Ilari, A.; Falvo, E.; Chiancone, E. (2003). "The Dps protein of Agrobacterium tumefaciens does not bind to DNA but protects it toward oxidative cleavage: x-ray crystal structure, iron binding, and hydroxyl-radical scavenging properties". The Journal of biological chemistry 278 (22): 20319–20326. doi:10.1074/jbc.M302114200. PMID 12660233. Ceci, P.; Ilari, A.; Falvo, E.; Chiancone, E. (2003). "The Dps protein of Agrobacterium tumefaciens does not bind to DNA but protects it toward oxidative cleavage: x-ray crystal structure, iron binding, and hydroxyl-radical scavenging properties". The Journal of biological chemistry 278 (22): 20319–20326. doi:10.1074/jbc.M302114200. PMID 12660233.
- ^ Nair, S.; Finkel, S. (2004). "Dps protects cells against multiple stresses during stationary phase". Journal of bacteriology 186 (13): 4192–4198. doi:10.1128/JB.186.13.4192-4198.2004. PMC 421617. PMID 15205421. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=421617. Nair, S.; Finkel, S. (2004). "Dps protects cells against multiple stresses during stationary phase". Journal of bacteriology 186 (13): 4192–4198. doi:10.1128/JB.186.13.4192-4198.2004. PMC 421617. PMID 15205421. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=421617.
- ^ Ilari, A.; Stefanini, S.; Chiancone, E.; Tsernoglou, D. (2000). "The dodecameric ferritin from Listeria innocua contains a novel intersubunit iron-binding site.". Nature structural biology 7 (1): 38–43. doi:10.1038/71236. PMID 10625425. Ilari, A.; Stefanini, S.; Chiancone, E.; Tsernoglou, D. (2000). "The dodecameric ferritin from Listeria innocua contains a novel intersubunit iron-binding site.". Nature structural biology 7 (1): 38–43. doi:10.1038/71236. PMID 10625425.
- ^ Allen, M.; Willits, D.; Mosolf, J.; Young, M.; Douglas, T. (2002). "Protein Cage Constrained Synthesis of Ferrimagnetic Iron Oxide Nanoparticles". Advanced Materials 14 (21): 1562–1565. doi:10.1002/1521-4095(20021104)14:21<1562::AID-ADMA1562>3.0.CO;2-D.
- ^ Allen, M.; Willits, D.; Young, M.; Douglas, T. (2003). "Constrained synthesis of cobalt oxide nanomaterials in the 12-subunit protein cage from Listeria innocua". Inorganic chemistry 42 (20): 6300–6305. doi:10.1021/ic0343657. PMID 14514305. Allen, M.; Willits, D.; Young, M.; Douglas, T. (2003). "Constrained synthesis of cobalt oxide nanomaterials in the 12-subunit protein cage from Listeria innocua". Inorganic chemistry 42 (20): 6300–6305. doi:10.1021/ic0343657. PMID 14514305.
- ^ Ceci, P.; Chiancone, E.; Kasyutich, O.; Bellapadrona, G.; Castelli, L.; Fittipaldi, M.; Gatteschi, D.; Innocenti, C. et al. (2010). "Synthesis of iron oxide nanoparticles in Listeria innocua Dps (DNA-binding protein from starved cells): a study with the wild-type protein and a catalytic centre mutant". Chemistry (Weinheim an der Bergstrasse, Germany) 16 (2): 709–717. doi:10.1002/chem.200901138. PMID 19859920. Ceci, P.; Chiancone, E.; Kasyutich, O.; Bellapadrona, G.; Castelli, L.; Fittipaldi, M.; Gatteschi, D.; Innocenti, C. et al. (2010). "Synthesis of iron oxide nanoparticles in Listeria innocua Dps (DNA-binding protein from starved cells): a study with the wild-type protein and a catalytic centre mutant". Chemistry (Weinheim an der Bergstrasse, Germany) 16 (2): 709–717. doi:10.1002/chem.200901138. PMID 19859920.
- ^ Prastaro, A.; Ceci, P.; Chiancone, E.; Boffi, A.; Cirilli, R.; Colone, M.; Fabrizi, G.; Stringaro, A. et al. (2009). "Suzuki-Miyaura cross-coupling catalyzed by protein-stabilized palladium nanoparticles under aerobic conditions in water: application to a one-pot chemoenzymatic enantioselective synthesis of chiral biaryl alcohols". Green Chemistry 11 (12): 1929. doi:10.1039/b915184b.
heme nonheme Metabolism: Metal metabolism Transition metal OtherZinc metabolismElectrolyte Sodium metabolismPhosphate metabolismMagnesium metabolismM: NUT
cof, enz, met
noco, nuvi, sysi/epon, met
drug(A8/11/12)
Categories:- Iron metabolism
Wikimedia Foundation. 2010.
