- Iodobenzene dichloride
-
Iodobenzene dichloride[1] 
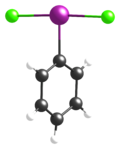 (Dichloro-λ3-iodanyl)benzeneOther namesIodosobenzene dichloride; Phenyliodine(III) dichloride; Phenyliodo dichloride; Phenyliodoso chloride; Phenylchloroiodonium chloride; Dichloroiodobenzene; Iododichlorobenzene
(Dichloro-λ3-iodanyl)benzeneOther namesIodosobenzene dichloride; Phenyliodine(III) dichloride; Phenyliodo dichloride; Phenyliodoso chloride; Phenylchloroiodonium chloride; Dichloroiodobenzene; IododichlorobenzeneIdentifiers Abbreviations IBD CAS number 932-72-9 
Jmol-3D images Image 1 - ClI(Cl)C1=CC=CC=C1
Properties Molecular formula C6H5Cl2I Molar mass 274.91 g mol−1 Appearance Yellow solid Density 2.2 g/cm3 Melting point 115–120 °C (dec.)
 dichloride (verify) (what is:
dichloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Iodobenzene dichloride (PhICl2) is a complex of iodobenzene with chlorine. It is used as an oxidant.
Single-crystal X-ray crystallography has been used to determine its structure; as can be predicted by VSEPR theory, it adopts a T-shaped geometry about the central iodine atom.[2][3]
Contents
Preparation
Iodobenzene dichloride is not stable, and is not commonly available commercially. It is prepared by passing chlorine gas through a solution of iodobenzene in chloroform, from which it precipitates.[4] The same reaction has been reported at pilot plant scale (20 kg) as well.[5]
- PhI + Cl2 → PhICl2
An alternate preparation involving the use of chlorine generated in situ by the action of sodium hypochlorite on hydrochloric acid has also been described.[6]
Reactions
Iodobenzene dichloride is hydrolyzed by basic solutions to give iodosobenzene (PhIO),[7] and is oxidized by sodium hypochlorite to give iodoxybenzene (PhIO2).[8]
In organic synthesis, iodobenzene dichloride is used as a reagent for the selective chlorination of alkenes[1] and alkynes.[9]
References
- ^ a b Phenyliodine(III) Dichloride, David W. Knight and Glen A. Russell, in Encyclopedia of Reagents for Organic Synthesis, 2001, John Wiley & Sons, Ltd doi:10.1002/047084289X.rp071
- ^ E. M. Archer and T. G. van Schalkwy (1953). "The crystal structure of benzene iododichloride". Acta Cryst. 6: 88–92. doi:10.1107/S0365110X53000193.
- ^ J. V. Carey, P. A. Chaloner, P. B. Hitchcock, T. Neugebauer, K. R. Seddon (1996). J. Chem. Res. 358: 2031–.
- ^ H. J. Lucas and E. R. Kennedy, "Iodobenzene dichloride", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0482; Coll. Vol. 3: 482
- ^ Zanka, Atsuhiko; Takeuchi, Hiroki; Kubota, Ariyoshi (1998). "Large-Scale Preparation of Iodobenzene Dichloride and Efficient Monochlorination of 4-Aminoacetophenone". Organic Process Research & Development 2 (4): 270. doi:10.1021/op980024e.
- ^ Zhao, Xue-Fei; Zhang, Chi (2007). "Iodobenzene Dichloride as a Stoichiometric Oxidant for the Conversion of Alcohols into Carbonyl Compounds; Two Facile Methods for Its Preparation". Synthesis 2007 (4): 551. doi:10.1055/s-2007-965889.
- ^ H. J. Lucas, E. R. Kennedy, and M. W. Formo (1955), "Iodosobenzene", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0483; Coll. Vol. 3: 483
- ^ M. W. Formo and John R. Johnson (1955), "Iodoxybenzene: B. Hypochlorite oxidation of iodobenzene dichloride", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0485; Coll. Vol. 3: 485
- ^ Michael E. Jung and Michael H. Parker (1997). "Synthesis of Several Naturally Occurring Polyhalogenated Monoterpenes of the Halomon Class". Journal of Organic Chemistry 62 (21): 7094–7095. doi:10.1021/jo971371. PMID 11671809.
Further reading
- Tanner, Dennis D; Van Bostelen, P. B. (1967). "Free-radical chlorination reactions of iodobenzene dichloride". Journal of Organic Chemistry 32 (5): 1517–1521. doi:10.1021/jo01280a047.
Categories:- Periodinanes
- Oxidizing agents
- Reagents for organic chemistry
Wikimedia Foundation. 2010.
