- Single-chain variable fragment
-
A single-chain variable fragment (scFv) is a fusion protein of the variable regions of the heavy (VH) and light chains (VL) of immunoglobulins, connected with a short linker peptide of ten to about 25 amino acids. The linker is usually rich in glycine for flexibility, as well as serine or threonine for solubility, and can either connect the N-terminus of the VH with the C-terminus of the VL, or vice versa.[1] This protein retains the specificity of the original immunoglobulin, despite removal of the constant regions and the introduction of the linker.[2] The image to the right shows how this modification usually leaves the specificity unaltered.
These molecules were created to facilitate phage display, where it is highly convenient to express the antigen-binding domain as a single peptide. As an alternative, scFv can be created directly from subcloned heavy and light chains derived from a hybridoma. ScFvs have many uses, e.g., flow cytometry, immunohistochemistry, and as antigen-binding domains of artificial T cell receptors.
Unlike monoclonal antibodies, which are often produced in mammalian cell cultures, scFvs are more often produced in bacteria cell cultures such as E. coli.[2]
Contents
Purification
Single-chain variable fragments lack the constant Fc region found in complete antibody molecules, and, thus, the common binding sites (e.g., Protein G) used to purify antibodies. These fragments can often be purified or immobilized using Protein L, since Protein L interacts with the variable region of kappa light chains. More commonly, scientists incorporate a six histidine tag on the c-terminus of the scFv molecule and purify them using immobilized metal affinity chromatography (IMAC). For unknown reasons, some scFv can also be captured by Protein A.
Bivalent and trivalent scFvs
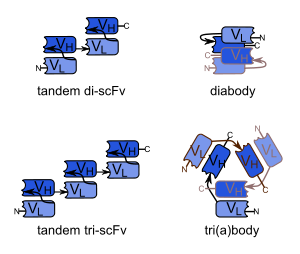
Divalent (or bivalent) single-chain variable fragments (di-scFvs, bi-scFvs) can be engineered by linking two scFvs. This can be done by producing a single peptide chain with two VH and two VL regions, yielding tandem scFvs.[3][4] Another possibility is the creation of scFvs with linker peptides that are too short for the two variable regions to fold together (about five amino acids), forcing scFvs to dimerize. This type is known as diabodies.[5] Diabodies have been shown to have dissociation constants up to 40-fold lower than corresponding scFvs, meaning that they have a much higher affinity to their target. Consequently, diabody drugs could be dosed much lower than other therapeutic antibodies and are capable of highly specific targeting of tumors in vivo.[6] Still shorter linkers (one or two amino acids) lead to the formation of trimers, so-called triabodies or tribodies. Tetrabodies have also been produced. They exhibit an even higher affinity to their targets than diabodies.[7]
All of these formats can be composed from variable fragments with specificity for two different antigens, in which case they are types of bispecific antibodies.[8][9] The furthest developed of these are bispecific tandem di-scFvs, known as bi-specific T-cell engagers (BiTEs).
Examples
- Pexelizumab, a scFv binding to component 5 of the complement system and used to reduce side effects of cardiac surgery[10]
- C6.5, a diabody targeting HER2/neu[6] found in some breast cancers
References
- ^ Schirrmann, Thomas (8 November 2004). "Tumorspezifisches Targeting der humanen Natürlichen Killerzellinie YT durch Gentransfer chimärer Immunglobulin-T-Zellrezeptoren" (in German). Berlin. http://edoc.hu-berlin.de/dissertationen/schirrmann-thomas-2005-03-18/HTML/.
- ^ a b Peterson, Eric; Owens, SM; Henry, RL (2006). "Monoclonal Antibody Form and Function: Manufacturing the Right Antibodies for Treating Drug Abuse". AAPS Journal 8 (2): E383–E390. doi:10.1208/aapsj080243. PMID 16796389. http://www.aapsj.org/view.asp?art=aapsj080243.
- ^ Xiong, Cheng-Yi; Natarajan, A; Shi, XB; Denardo, GL; Denardo, SJ (2006). "Development of tumor targeting anti-MUC-1 multimer: effects of di-scFv unpaired cysteine location on PEGylation and tumor binding". Protein Engineering Design and Selection 19 (8): 359–367. doi:10.1093/protein/gzl020. PMID 16760193. http://peds.oxfordjournals.org/cgi/content/full/19/8/359.
- ^ Kufer, Peter; Lutterbüse, Ralf; Baeuerle, Patrick A. (2004). "A revival of bispecific antibodies". Trends in Biotechnology 22 (5): 238–244. doi:10.1016/j.tibtech.2004.03.006. PMID 15109810. http://www.micromet.de/fileadmin/template/main/pdf/publications_147881aaf61df52304237f0ee7f0cf2a.pdf.
- ^ Hollinger, Philipp; Prospero, T; Winter, G (July 1993). ""Diabodies": small bivalent and bispecific antibody fragments". Proceedings of the National Academy of Sciences of the United States of America 90 (14): 6444–8. PMC 46948. PMID 8341653. http://www.ncbi.nlm.nih.gov/pubmed/8341653.
- ^ a b Adams, GP; Schier, R; McCall, AM; Crawford, RS; Wolf, EJ; Weiner, LM; Marks, JD (1998). "Prolonged in vivo tumour retention of a human diabody targeting the extracellular domain of human HER2/neu". British journal of cancer 77 (9): 1405–12. doi:10.1038/bjc.1998.233. PMC 2150193. PMID 9652755. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2150193.
- ^ Le Gall, F.; Kipriyanov, SM; Moldenhauer, G; Little, M (1999). "Di-, tri- and tetrameric single chain Fv antibody fragments against human CD19: effect of valency on cell binding". FEBS Letters 453 (1): 164–168. doi:10.1016/S0014-5793(99)00713-9. PMID 10403395.
- ^ Dincq, S; Bosman, F; Buyse, MA; Degrieck, R; Celis, L; De Boer, M; Van Doorsselaere, V; Sablon, E (2001). "Expression and purification of monospecific and bispecific recombinant antibody fragments derived from antibodies that block the CD80/CD86-CD28 costimulatory pathway". Protein expression and purification 22 (1): 11–24. doi:10.1006/prep.2001.1417. PMID 11388794.
- ^ Kellner, C (2008). "Entwicklung und Charakterisierung bispezifischer Antikörper-Derivate zur Immuntherapie CD19-positiver Leukämien und Lymphome [Development and characterisation of bispecific antibody derivatives for the immunotherapy of CD19-positive leukaemia and lymphoma]" (in German and English). Erlangen-Nürnberg: Friedrich-Alexander-Universität. http://www.opus.ub.uni-erlangen.de/opus/volltexte/2009/1235/.
- ^ Mathew, JP; Shernan, SK; White, WD; Fitch, JC; Chen, JC; Bell, L; Newman, MF (2004). "Preliminary report of the effects of complement suppression with pexelizumab on neurocognitive decline after coronary artery bypass graft surgery". Stroke; a journal of cerebral circulation 35 (10): 2335–9. doi:10.1161/01.STR.0000141938.00524.83. PMID 15331798.
Engineered monoclonal antibodies and antibody mimetics Whole antibody bispecific: Trifunctional antibody
Fab fragment Variable fragment Single-chain variable fragment / di-scFv / tri-scFv • Single-domain antibody • Small modular immunopharmaceutical • bispecific: T-cell engagerSmaller units Intracellular IntrabodyAntibody mimetics Categories:
Wikimedia Foundation. 2010.