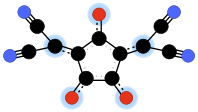
- Croconate violet
-
Croconate violet or 1,3-bis(dicyanomethylene)croconate is a divalent anion with chemical formula C11N4O2−
3 or ((N≡C-)2C=)2(C5O3)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C5O2−
5 through the replacement of two oxygen atoms by dicyanomethylene groups =C(-C≡N)2. Its systematic name is 3,5-bis(dicyanomethylene)-1,2,4-trionate. The term croconate violet as a dye name specifically refers to the dipotassium salt K2C11N4O3.Contents
History and synthesis
The anion was syntesized and characterized by A. Fatiadi in 1978. He obtained the potassium salt by treating dipotassium croconate K2C5O5 with malononitrile in water solution at 80–90 °C.[1] The dipotassium salt crystallyzes from water as a dihydrate in deep blue metallic needles. The water solutions have an intense violet color and strongly stain the skin. [1]
Croconic acid violet
The croconate violet anion is the conjugate base of the acid croconic acid violet C11H2N4O3, also obtained by Fatiadi in 1978 by treating the potassium salt with hydrochloric acid. It is an orange crystalline solid that melts at 260–270°C and dissolves in water to give violet skin-staining solutions. It strongly acidic (pK1 = 0.32 ± 0.02, pK2 = 1.02 ± 0.02). From this acid other alkali metal salts can be prepared. The acid cannot be prepared directly from croconic acid and malononitrile; croconate blue is obtained instead.[1]
Properties
Croconate violet salts are dyes with strong absorptions in the UV-Vis region. Solutions of the acid or of the dipotassium salt strongly stain the skin. [1] It retains the aromatic character and some other properties of the croconate anion.[2]
Croconate violet salts also have interesting electrochemical, semiconducting and photophysical properties, and have been the subject of research in supramolecular chemistry.[3] The dipotassium salt for example is a semiconductor with electrical conductivity 0.0002 Ω-1 cm-1.[1]
The lithium salt is very soluble in water.[1] The rubidium salt Rb2C11N4O3 and the mixed rubidium-potassium salt Rb1.4K0.6C11N4O3 crystallyze as deep blue dihydrates in the triclinic space group, with the same structure. In these solids the croconate violet anion is almost planar, with the dicyano methylene groups slightly twisted out of the mean plane. The aromatic character of the croconate core is retained or even enhanced, with strongly delocalized π electrons. The lanthanides cations (lanthanum, neodymium, gadolinium, and holmium) exhibit some degree of π-stacking interactions; namely, the π electrons of the croconate rings in one layer form weak bonds with those in the adjacent layers.[5] In the anhydrous tetrabutylammonium salt the anions are separated by more than 12 Å and no π-stacking occurs.[4]
See also
- 2-(dicyanomethylene)croconate
- Croconate blue, 1,2,3-tris(dicyanomethylene)croconate
- 1,2-bis(dicyanomethylene)squarate
- 1,3-bis(dicyanomethylene)squarate
References
- ^ a b c d e f Alexander J. Fatiadi (1978), Synthesis of 1,3-(dicyanomethylene)croconate salts. New bond-delocalized dianion, "Croconate Violet". Journal of the American Chemical Society, volume 100 issue 8, pages 2586–2587. doi:10.1021/ja00476a073
- ^ Lawrence M. Doane, Alexander J. Fatiadi (2003) Electrochemical Oxidation of Croconate Salts; Evidence of the Chemical Equivalence of the Carbonyl Oxygen Atom and the Dicyanomethylene Group doi:10.1002/anie.198206351
- ^ Renata Diniz, Lívian R.V. De Sá, Bernardo L. Rodrigues, Maria I. Yoshida and Luiz Fernando C. de Oliveira (2006), Crystal structure and vibrational spectra of rubidium salts of bis(dicyanomethylene)croconate (croconate violet). Inorganica Chimica Acta, Volume 359, Issue 7, Pages 2296-2302. doi:10.1016/j.ica.2006.01.012
- ^ a b Wagner M. Teles, Regis de A. Farani, Daniel S. Maia, Nivaldo L. Speziali, Maria Irene Yoshida, Luiz Fernando C. de Oliveira and Flávia C. Machado (2006), Crystal structure, thermal analysis and spectroscopic properties of Tetrabutylammonium 3,5-bis(dicyanomethylene)-cyclopentane-1,2,4-trionate: An intriguing pseudo-oxocarbon and its zinc(II) complex. Journal of Molecular Structure, Volume 783, Issues 1-3, Pages 52-60 doi:10.1016/j.molstruc.2005.08.020
- ^ Luiz Felipe O. Faria, Antônio L. Soares Jr., Renata Diniz, Maria I. Yoshida, Howell G.M. Edwards and Luiz Fernando C. de Oliveira (2010), Mixed salts containing croconate violet, lanthanide and potassium ions: Crystal structures and spectroscopic characterization of supramolecular compounds. Inorganica Chimica Acta, Volume 363, Issue 1, Pages 49-56 doi:10.1016/j.ica.2009.09.050
Categories:- Dyes
- Oxocarbons
Wikimedia Foundation. 2010.