- Cobalamin biosynthesis
-
CobD_Cbib Identifiers Symbol CobD_Cbib Pfam PF03186 InterPro IPR004485 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CobS Identifiers Symbol CobS Pfam PF02654 InterPro IPR003805 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CobT Identifiers Symbol CobT Pfam PF06213 InterPro IPR006538 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CobU 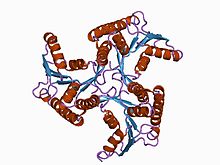
adenosylcobinamide kinase/adenosylcobinamide phosphate guanylyltransferase (cobu) from salmonella typhimurium Identifiers Symbol CobU Pfam PF02283 Pfam clan CL0023 InterPro IPR003203 SCOP 1cbu Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary cobW 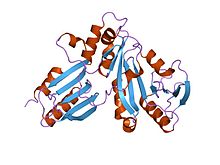
yjia protein Identifiers Symbol cobW Pfam PF02492 Pfam clan CL0023 InterPro IPR003495 SCOP 1nij Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CobW C terminal 
yjia protein Identifiers Symbol CobW_C Pfam PF07683 InterPro IPR011629 SCOP 1nij Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CbiA 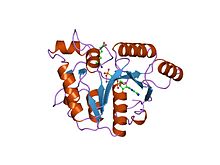
dethiobiotin synthetase complexed with 7,8-diamino-nonanoic acid, 5'-adenosyl-methylene-triphosphate, and manganese Identifiers Symbol CbiA Pfam PF01656 Pfam clan CL0023 InterPro IPR002586 SCOP 1dts Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CbiD 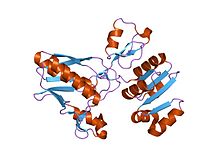
structural genomics, 1.9a crystal structure of cobalamin biosynthesis protein (cbid) from archaeoglobus fulgidus Identifiers Symbol CbiD Pfam PF01888 InterPro IPR002748 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CbiG N terminus Identifiers Symbol CbiG_N Pfam PF11760 InterPro IPR021744 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CbiG central region Identifiers Symbol CbiG_mid Pfam PF11761 InterPro IPR021745 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CbiG C terminus Identifiers Symbol CbiG_C Pfam PF01890 InterPro IPR002750 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CbiJ Identifiers Symbol CbiJ Pfam PF02571 InterPro IPR003723 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CbiM Identifiers Symbol CbiM Pfam PF01891 Pfam clan CL0315 InterPro IPR002751 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CbiN Identifiers Symbol CbiN Pfam PF02553 InterPro IPR003705 TCDB 3.A.1 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CbiQ Identifiers Symbol CbiQ Pfam PF02361 InterPro IPR003339 TCDB 3.A.1 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CbiX 
crystal structure of hypothetical protein af0721 from archaeoglobus fulgidus Identifiers Symbol CbiX Pfam PF01903 Pfam clan CL0043 InterPro IPR002762 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary CbiZ Identifiers Symbol CbiZ Pfam PF01955 InterPro IPR002808 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary In molecular biology, cobalamin biosynthesis is the synthesis of cobalamin (vitamin B12).
Contents
Cobalamin
Cobalamin (vitamin B12) is a structurally complex cofactor, consisting of a modified tetrapyrrole with a centrally chelated cobalt. Cobalamin is usually found in one of two biologically active forms: methylcobalamin and adocobalamin. Most prokaryotes, as well as animals, have cobalamin-dependent enzymes, whereas plants and fungi do not appear to use it. In bacteria and archaea, these include methionine synthase, ribonucleotide reductase, glutamate and methylmalonyl-CoA mutases, ethanolamine ammonia lyase, and diol dehydratase.[1] In mammals, cobalamin is obtained through the diet, and is required for methionine synthase and methylmalonyl-CoA mutase.[2]
Pathways of cobalamin biosynthesis
There are at least two distinct cobalamin biosynthetic pathways in bacteria:[3]
- Aerobic pathway that requires oxygen and in which cobalt is inserted late in the pathway;[4] found in Pseudomonas denitrificans and Rhodobacter capsulatus.
- Anaerobic pathway in which cobalt insertion is the first committed step towards cobalamin synthesis;[5] found in Salmonella typhimurium, Bacillus megaterium, and Propionibacterium freudenreichii subsp. shermanii.
Either pathway can be divided into two parts:
- Corrin ring synthesis (differs in aerobic and anaerobic pathways)
- Adenosylation of corrin ring, attachment of aminopropanol arm, and assembly of the nucleotide loop (common to both pathways).[6]
Proteins involved in cobalamin biosynthesis
There are about 30 enzymes involved in either pathway, where those involved in the aerobic pathway are prefixed Cob and those of the anaerobic pathway Cbi. Several of these enzymes are pathway-specific: CbiD, CbiG, and CbiK are specific to the anaerobic route of S. typhimurium, whereas CobE, CobF, CobG, CobN, CobS, CobT, and CobW are unique to the aerobic pathway of P. denitrificans.
CbiB/CobD
The CbiB or CobD protein converts cobyric acid to cobinamide by the addition of aminopropanol on the F carboxylic group. It is part of the cob operon.[7]
CobT/CobN/CobS
Aerobic cobalt chelatase consists of three subunits, CobT, CobN and CobS. Cobalamin (vitamin B12) can be complexed with metal via the ATP-dependent reactions (aerobic pathway) (e.g., in P. denitrificans) or via ATP-independent reactions (anaerobic pathway) (e.g., in Salmonella typhimurium).[8][9] The corresponding cobalt chelatases are not homologous. However, aerobic cobalt chelatase subunits CobN and CobS are homologous to Mg-chelatase subunits BchH and BchI, respectively.[9] CobT, too, has been found to be remotely related to the third subunit of Mg-chelatase, BchD (involved in bacteriochlorophyll synthesis, e.g., in Rhodobacter capsulatus).[9]
The CobS protein is a cobalamin-5-phosphate synthase that catyalzes the reactions:
- Adenosylcobinamide-GDP + alpha-ribazole-5'-P = adenosylcobalamin-5'-phosphate + GMP
- Adenosylcobinamide-GDP + alpha-ribazole = adenosylcobalamin + GMP
The protein product from these catalyses is associated with a large complex of proteins and is induced by cobinamide. CobS is involved in part III of cobalamin biosynthesis, one of the late steps in adenosylcobalamin synthesis that, together with CobU, CobT, and CobC proteins, defines the nucleotide loop assembly pathway.[10][11]
CobU
CobU proteins are bifunctional cobalbumin biosynthesis enzymes which display cobinamide kinase and cobinamide phosphate guanyltransferase activity. The crystal structure of the enzyme reveals the molecule to be a trimer with a propeller-like shape.[12]
CobW
CobW proteins are generally found proximal to the trimeric cobaltochelatase subunit CobN, which is essential for vitamin B12 (cobalamin) biosynthesis.[1] They contain a P-loop nucleotide-binding loop in the N-terminal domain and a histidine-rich region in the C-terminal portion suggesting a role in metal binding, possibly as an intermediary between the cobalt transport and chelation systems. CobW might be involved in cobalt reduction leading to cobalt(I) corrinoids. CobW-like proteins include P47K, a Pseudomonas chlororaphis protein needed for nitrile hydratase expression,[13] and urease accessory protein UreG, which acts as a chaperone in the activation of urease upon insertion of nickel into the active site.[14]
CbiA
The CbiA family of proteins consists of various cobyrinic acid a,c-diamide synthases. These include CbiA and CbiP from Salmonella typhimurium.,[15] and CobQ from Rhodobacter capsulatus.[16] These amidases catalyse amidations to various side chains of hydrogenobyrinic acid or cobyrinic acid a,c-diamide in the biosynthesis of cobalamin (vitamin B12) from uroporphyrinogen III.[15]
CbiD
CbiD is an essential protein for cobalamin biosynthesis in both Salmonella typhimurium and Bacillus megaterium. A deletion mutant of CbiD suggests that this enzyme is involved in C-1 methylation and deacylation reactions required during the ring contraction process in the anaerobic pathway to cobalamin (similar role as CobF).[17] The CbiD protein has a putative S-AdoMet binding site.[18] CbiD has no counterpart in the aerobic pathway.
CbiG
CbiG proteins are specific for anaerobic cobalamin biosynthesis. CbiG, which shows homology with CobE of the aerobic pathway, participates in the conversion of cobalt-precorrin 5 into cobalt-precorrin 6.[19] CbiG is responsible for the opening of the delta-lactone ring and extrusion of the C2-unit.[20] The aerobic pathway uses molecular oxygen to trigger the events at C-20 leading to contraction and expulsion of the C2-unit as acetic acid from a metal-free intermediate, whereas the anaerobic route involves the internal delivery of oxygen from a carboxylic acid terminus to C-20 followed by extrusion of the C2-unit as acetaldehyde, using cobalt complexes as substrates.[20]
CbiJ
The CbiJ family of proteins includes the CobK and CbiJ precorrin-6x reductases EC 1.3.1.54. In the aerobic pathway, CobK catalyses the reduction of the macrocycle of precorrin-6X to produce precorrin-6Y; while in the anaerobic pathway CbiJ catalyses the reduction of the macrocycle of cobalt-precorrin-6X into cobalt-precorrin-6Y.[21][22]
CbiM
CbiM is an integral membrane protein which is involved in cobalamin synthesis, its exact function in unknown.
CbiN
The cobalt transport protein CbiN is part of the active cobalt transport system involved in uptake of cobalt in to the cell involved with cobalamin biosynthesis (vitamin B12). It has been suggested that CbiN may function as the periplasmic binding protein component of the active cobalt transport system.[16]
CbiQ
The CbiQ family consists of various cobalt transport proteins Most of which are found in Cobalamin (Vitamin B12) biosynthesis operons. In Salmonella the cbiN cbiQ (product CbiQ in this family) and cbiO are likely to form an active cobalt transport system.[23]
CbiX
The CbiX protein functions as a cobalt-chelatase in the anaerobic biosynthesis of cobalamin. It catalyses the insertion of cobalt into sirohydrochlorin. The structure of CbiX from Archaeoglobus fulgidus consists of a central mixed beta-sheet flanked by four alpha-helices, although it is about half the size of other Class II tetrapyrrole chelatases.[24] The CbiX proteins found in archaea appear to be shorter than those found in eubacteria.[25]
CbiZ
The CbiZ family of proteins includes CbiZ, which is involved in the salvage pathway of cobinamide in archaea. Archaea convert adenosylcobinamide (AdoCbi) into adenosylcobinamide phosphate (AdoCbi-P) in two steps. First, the amidohydrolase activity of CbiZ cleaves off the aminopropanol moiety of AdoCbi yielding adenosylcobyric acid (AdoCby); second, AdoCby is converted into AdoCbi-P by the action of adenosylcobinamide-phosphate synthase (CbiB). Adenosylcobyric acid is an intermediate of the de novo coenzyme B12 biosynthetic route.[26]
References
- ^ a b Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS (October 2003). "Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes". J. Biol. Chem. 278 (42): 41148–59. doi:10.1074/jbc.M305837200. PMID 12869542.
- ^ Banerjee R (April 2006). "B12 trafficking in mammals: A for coenzyme escort service". ACS Chem. Biol. 1 (3): 149–59. doi:10.1021/cb6001174. PMID 17163662.
- ^ Roessner CA, Santander PJ, Scott AI (2001). "Multiple biosynthetic pathways for vitamin B12: variations on a central theme". Vitam. Horm. 61: 267–97. PMID 11153269.
- ^ Heldt D, Lawrence AD, Lindenmeyer M, Deery E, Heathcote P, Rigby SE, Warren MJ (August 2005). "Aerobic synthesis of vitamin B12: ring contraction and cobalt chelation". Biochem. Soc. Trans. 33 (Pt 4): 815–9. doi:10.1042/BST0330815. PMID 16042605.
- ^ Roessner CA, Huang KX, Warren MJ, Raux E, Scott AI (June 2002). "Isolation and characterization of 14 additional genes specifying the anaerobic biosynthesis of cobalamin (vitamin B12) in Propionibacterium freudenreichii (P. shermanii)". Microbiology (Reading, Engl.) 148 (Pt 6): 1845–53. PMID 12055304.
- ^ Raux E, Schubert HL, Warren MJ (December 2000). "Biosynthesis of cobalamin (vitamin B12): a bacterial conundrum". Cell. Mol. Life Sci. 57 (13-14): 1880–93. doi:10.1007/PL00000670. PMID 11215515.
- ^ Woodson JD, Zayas CL, Escalante-Semerena JC (December 2003). "A new pathway for salvaging the coenzyme B12 precursor cobinamide in archaea requires cobinamide-phosphate synthase (CbiB) enzyme activity". J. Bacteriol. 185 (24): 7193–201. PMC 296239. PMID 14645280. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=296239.
- ^ Roth JR, Lawrence JG, Bobik TA (1996). "Cobalamin (coenzyme B12): synthesis and biological significance". Annu. Rev. Microbiol. 50: 137–81. doi:10.1146/annurev.micro.50.1.137. PMID 8905078.
- ^ a b c Fodje MN, Hansson A, Hansson M, Olsen JG, Gough S, Willows RD, Al-Karadaghi S (August 2001). "Interplay between an AAA module and an integrin I domain may regulate the function of magnesium chelatase". J. Mol. Biol. 311 (1): 111–22. doi:10.1006/jmbi.2001.4834. PMID 11469861.
- ^ Maggio-Hall LA, Escalante-Semerena JC (October 1999). "In vitro synthesis of the nucleotide loop of cobalamin by Salmonella typhimurium enzymes". Proc. Natl. Acad. Sci. U.S.A. 96 (21): 11798–803. doi:10.1073/pnas.96.21.11798. PMC 18366. PMID 10518530. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=18366.
- ^ Maggio-Hall LA, Claas KR, Escalante-Semerena JC (May 2004). "The last step in coenzyme B(12) synthesis is localized to the cell membrane in bacteria and archaea". Microbiology (Reading, Engl.) 150 (Pt 5): 1385–95. PMID 15133100.
- ^ Thompson TB, Thomas MG, Escalante-Semerena JC, Rayment I (May 1998). "Three-dimensional structure of adenosylcobinamide kinase/adenosylcobinamide phosphate guanylyltransferase from Salmonella typhimurium determined to 2.3 A resolution,". Biochemistry 37 (21): 7686–95. doi:10.1021/bi973178f. PMID 9601028.
- ^ Hashimoto Y, Nishiyama M, Horinouchi S, Beppu T (October 1994). "Nitrile hydratase gene from Rhodococcus sp. N-774 requirement for its downstream region for efficient expression". Biosci. Biotechnol. Biochem. 58 (10): 1859–65. doi:10.1271/bbb.58.1859. PMID 7765511.
- ^ Zambelli B, Musiani F, Savini M, Tucker P, Ciurli S (March 2007). "Biochemical studies on Mycobacterium tuberculosis UreG and comparative modeling reveal structural and functional conservation among the bacterial UreG family". Biochemistry 46 (11): 3171–82. doi:10.1021/bi6024676. PMID 17309280.
- ^ a b Pollich M, Klug G (August 1995). "Identification and sequence analysis of genes involved in late steps in cobalamin (vitamin B12) synthesis in Rhodobacter capsulatus". J. Bacteriol. 177 (15): 4481–7. PMC 177200. PMID 7635831. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=177200.
- ^ a b Roth JR, Lawrence JG, Rubenfield M, Kieffer-Higgins S, Church GM (June 1993). "Characterization of the cobalamin (vitamin B12) biosynthetic genes of Salmonella typhimurium". J. Bacteriol. 175 (11): 3303–16. PMC 204727. PMID 8501034. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=204727.
- ^ Roessner CA, Williams HJ, Scott AI (April 2005). "Genetically engineered production of 1-desmethylcobyrinic acid, 1-desmethylcobyrinic acid a,c-diamide, and cobyrinic acid a,c-diamide in Escherichia coli implies a role for CbiD in C-1 methylation in the anaerobic pathway to cobalamin". J. Biol. Chem. 280 (17): 16748–53. doi:10.1074/jbc.M501805200. PMID 15741157.
- ^ Raux E, Lanois A, Warren MJ, Rambach A, Thermes C (October 1998). "Cobalamin (vitamin B12) biosynthesis: identification and characterization of a Bacillus megaterium cobI operon". Biochem. J. 335 ( Pt 1): 159–66. PMC 1219764. PMID 9742225. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1219764.
- ^ Scott AI, Roessner CA (August 2002). "Biosynthesis of cobalamin (vitamin B(12))". Biochem. Soc. Trans. 30 (4): 613–20. PMID 12196148.
- ^ a b Kajiwara Y, Santander PJ, Roessner CA, Pérez LM, Scott AI (August 2006). "Genetically engineered synthesis and structural characterization of cobalt-precorrin 5A and -5B, two new intermediates on the anaerobic pathway to vitamin B12: definition of the roles of the CbiF and CbiG enzymes". J. Am. Chem. Soc. 128 (30): 9971–8. doi:10.1021/ja062940a. PMID 16866557.
- ^ Kim W, Major TA, Whitman WB (December 2005). "Role of the precorrin 6-X reductase gene in cobamide biosynthesis in Methanococcus maripaludis". Archaea 1 (6): 375–84. PMC 2685584. PMID 16243778. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2685584.
- ^ Shearer N, Hinsley AP, Van Spanning RJ, Spiro S (November 1999). "Anaerobic growth of Paracoccus denitrificans requires cobalamin: characterization of cobK and cobJ genes". J. Bacteriol. 181 (22): 6907–13. PMC 94164. PMID 10559155. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=94164.
- ^ Roth, J. R.; Lawrence, J. G.; Rubenfield, M.; Kieffer-Higgins, S.; Church, G. M. (1993). "Characterization of the cobalamin (vitamin B12) biosynthetic genes of Salmonella typhimurium". Journal of bacteriology 175 (11): 3303–3316. PMC 204727. PMID 8501034. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=204727.
- ^ Yin J, Xu LX, Cherney MM, Raux-Deery E, Bindley AA, Savchenko A, Walker JR, Cuff ME, Warren MJ, James MN (March 2006). "Crystal structure of the vitamin B12 biosynthetic cobaltochelatase, CbiXS, from Archaeoglobus fulgidus". J. Struct. Funct. Genomics 7 (1): 37–50. doi:10.1007/s10969-006-9008-x. PMID 16835730.
- ^ Brindley AA, Raux E, Leech HK, Schubert HL, Warren MJ (June 2003). "A story of chelatase evolution: identification and characterization of a small 13-15-kDa "ancestral" cobaltochelatase (CbiXS) in the archaea". J. Biol. Chem. 278 (25): 22388–95. doi:10.1074/jbc.M302468200. PMID 12686546.
- ^ Woodson JD, Escalante-Semerena JC (March 2004). "CbiZ, an amidohydrolase enzyme required for salvaging the coenzyme B12 precursor cobinamide in archaea". Proc. Natl. Acad. Sci. U.S.A. 101 (10): 3591–6. doi:10.1073/pnas.0305939101. PMC 373507. PMID 14990804. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=373507.
This article includes text from the public domain Pfam and InterPro IPR004485
This article includes text from the public domain Pfam and InterPro IPR003805
This article includes text from the public domain Pfam and InterPro IPR006538
This article includes text from the public domain Pfam and InterPro IPR003203
This article includes text from the public domain Pfam and InterPro IPR003495
This article includes text from the public domain Pfam and InterPro IPR011629
This article includes text from the public domain Pfam and InterPro IPR002586
This article includes text from the public domain Pfam and InterPro IPR002748
This article includes text from the public domain Pfam and InterPro IPR002750
This article includes text from the public domain Pfam and InterPro IPR003723
This article includes text from the public domain Pfam and InterPro IPR002751
This article includes text from the public domain Pfam and InterPro IPR003705
This article includes text from the public domain Pfam and InterPro IPR003339
This article includes text from the public domain Pfam and InterPro IPR002762
This article includes text from the public domain Pfam and InterPro IPR002808
Categories:- Protein families
- Vitamin B12
- Biosynthesis
Wikimedia Foundation. 2010.
