- Chloro(dimethyl sulfide)gold(I)
-
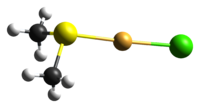
Chloro(dimethyl sulfide)gold(I) 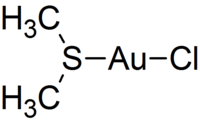
Identifiers CAS number 29892-37-3 Properties Molecular formula C2H6AuClS Molar mass 294.55 g mol−1 Hazards EU classification  Xi
XiR-phrases R36/37/38 S-phrases S26-S36 Related compounds Related compounds chloro(tetrahydrothiophene)gold(I)  sulfide)gold(I) (verify) (what is:
sulfide)gold(I) (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chloro(dimethyl sulfide)gold(I) is an coordination complex of gold. It is a white solid. This compound is a common entry point into gold chemistry.
Contents
Structure
As for many other gold(I) complexes, the compound adopts a nearly linear (176.9°) geometry about the central gold centre. The Au-S bond distance is 2.271(2) Å, which is similar to other gold(I)-sulfur bonds.[1]
Preparation
Chloro(dimethyl sulfide)gold(I) is commercially available. It may be prepared by dissolving gold in aqua regia (to give chloroauric acid), followed by addition of dimethyl sulfide.[2] Alternatively, sodium tetrachloroaurate may be used as the source of gold(III).[3] The bromo analog, Me2SAuBr, has also been synthesized by a similar route.[4] An approximate equation is:
- HAuCl4 + 2 SMe2 + H2O → Me2SAuCl + 3 HCl + OSMe2
Reactions
In chloro(dimethyl sulfide)gold(I), the dimethyl sulfide ligand is easily displaced by other ligands:
- Me2SAuCl + L → LAuCl + Me2S (L = ligand)
Since Me2S is volatile, the new complex LAuCl is often easily purified.
When exposed to light and air, the compound decomposes to elemental gold.
References
- ^ P. G. Jones and J. Lautner (1988). "Chloro(dimethyl sulfide)gold(I)". Acta Cryst. C 44 (12): 2089–2091. doi:10.1107/S0108270188009151.
- ^ Marie-Claude Brandys , Michael C. Jennings and Richard J. Puddephatt (2000). "Luminescent gold(I) macrocycles with diphosphine and 4,4-bipyridyl ligands". J. Chem. Soc., Dalton Trans. (24): 4601–4606. doi:10.1039/b005251p.
- ^ Nishina, Naoko; Yamamoto, Yoshinori (2007). "Gold-Catalyzed Intermolecular Hydroamination of Allenes: First Example of the Use of an Aliphatic Amine in Hydroamination". Synlett 2007 (11): 1767. doi:10.1055/s-2007-984501.
- ^ Hickey, James L.; Ruhayel, Rasha A.; Barnard, Peter J.; Baker, Murray V.; Berners-Price, Susan J.; Filipovska, Aleksandra (2008). "Mitochondria-Targeted Chemotherapeutics: The Rational Design of Gold(I)N-Heterocyclic Carbene Complexes That Are Selectively Toxic to Cancer Cells and Target Protein Selenols in Preference to Thiols". J. Am. Chem. Soc. 130 (38): 12570. doi:10.1021/ja804027j. PMID 18729360.
Categories:- Gold compounds
- Chlorides
- Organosulfur compounds
Wikimedia Foundation. 2010.
