- Ethylenediaminetetraacetic acid
-
"EDTA" redirects here. For other uses, see EDTA (disambiguation).
Ethylenediaminetetraacetic acid 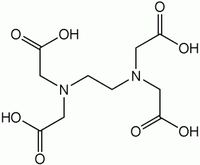
 2,2',2'',2'''-(Ethane-1,2-diyldinitrilo)tetraacetic acidSystematic name2-({2-[bis(carboxymethyl)amino]ethyl}(carboxymethyl)amino)acetic acidOther namesDiaminoethane-tetraacetic acid
2,2',2'',2'''-(Ethane-1,2-diyldinitrilo)tetraacetic acidSystematic name2-({2-[bis(carboxymethyl)amino]ethyl}(carboxymethyl)amino)acetic acidOther namesDiaminoethane-tetraacetic acid
Edetic acid
Ethylenedinitrilo-tetraacetic acid
VerseneIdentifiers Abbreviations EDTA
H4EDTACAS number 60-00-4  , 15251-22-6 (2H),(2H),(2H)
, 15251-22-6 (2H),(2H),(2H)PubChem 6049  , 46781544 (13C),(13C),(1-13C)
, 46781544 (13C),(13C),(1-13C)  , 16217600 (2H),(2H),(2H)
, 16217600 (2H),(2H),(2H) 
ChemSpider 5826  , 17345117 (2H),(2H),(2H)
, 17345117 (2H),(2H),(2H) 
UNII 9G34HU7RV0  [U.S.FDA]
[U.S.FDA]EC number 200-449-4 UN number 3077 DrugBank DB00974 KEGG D00052 
MeSH Edetic+acid ChEBI CHEBI:42191 
ChEMBL CHEMBL858 
RTECS number AH4025000 ATC code V03 Beilstein Reference 1716295 Jmol-3D images Image 1 - C(CN(CC(=O)O)CC(=O)O)N(CC(=O)O)CC(=O)O
Properties Molecular formula C10H16N2O8 Molar mass 292.24 g mol−1 Density 0.86 g cm−3 Melting point 237-245 °C, 510-518 K, 459-473 °F (dec.)
Acidity (pKa) pKa1 = 0.0 (CO2H) (µ = 1.0)
pKa2 = 1.5 (CO2H) (µ = 0.1)
pKa3 = 2.00 (CO2H) (µ = 0.1)
pKa4 = 2.69 (CO2H) (µ = 0.1)
pKa5 = 6.13 (NH+) (µ = 0.1)
pKa6 = 10.37 (NH+) (µ = 0.1)[1]Hazards MSDS External MSDS R-phrases R36 S-phrases S26 Main hazards irritant NFPA 704  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Ethylenediaminetetraacetic acid, widely abbreviated as EDTA (for other names, see Table), is a polyamino carboxylic acid and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ("six-toothed") ligand and chelating agent, i.e. its ability to "sequester" metal ions such as Ca2+ and Fe3+. After being bound by EDTA, metal ions remain in solution but exhibit diminished reactivity. EDTA is produced as several salts, notably disodium EDTA and calcium disodium EDTA.
Contents
Synthesis
The compound was first described in 1935 by Ferdinand Munz, who prepared the compound from ethylenediamine and chloroacetic acid.[2] Today, EDTA is mainly synthesised from ethylenediamine (1,2-diaminoethane), formaldehyde, and sodium cyanide.[3] This route yields the sodium salt, which can be converted in a subsequent step into the acid forms:
- H2NCH2CH2NH2 + 4 CH2O + 4 NaCN + 4 H2O → (NaO2CCH2)2NCH2CH2N(CH2CO2Na)2 + 4 NH3
- (NaO2CCH2)2NCH2CH2N(CH2CO2Na)2 + 4 HCl → (HO2CCH2)2NCH2CH2N(CH2CO2H)2 + 4 NaCl
In this way, about 80M kilograms are produced each year. Impurities cogenerated by this route include glycine and nitrilotriacetic acid; they arise from reactions of the ammonia coproduct.[4]
Nomenclature
To describe EDTA and its various protonated forms, chemists distinguish between EDTA4−, the conjugate base that is the ligand, and H4EDTA, the precursor to that ligand. At very low pH (very acidic conditions) the fully protonated H6EDTA2+ form predominates, whereas at very high pH or very basic condition, the fully deprotonated Y4− form is prevalent. In this article, the term EDTA is used to mean H4-xEDTAx-, whereas in its complexes EDTA4- stands for the tetra-deprotonated ligand.
Coordination chemistry principles
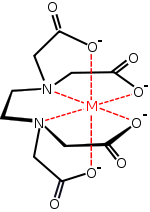
In coordination chemistry, EDTA4- is a member of the polyamino carboxylic acid family of ligands. EDTA4- usually binds to a metal cation through its two amines and four carboxylates. Many of the resulting coordination compounds adopt octahedral geometry. Although of little consequence for its applications, these octahedral complexes are chiral. The anion [Co(EDTA)]− has been resolved into enantiomers.[5] Many complexes of EDTA4- adopt more complex structures due to (i) the formation of an additional bond to water, i.e. seven-coordinate complexes, or (ii) the displacement of one carboxylate arm by water. Early work on the development of EDTA was undertaken by Gerold Schwarzenbach in the 1940s.[6] EDTA forms especially strong complexes with Mn(II), Cu(II), Fe(III), Pb (II) and Co(III).[7]
Several features of EDTA's complexes are relevant to its applications. First, because of its high denticity, this ligand has a high affinity for metal cations:
- [Fe(H2O)6]3+ + H4EDTA
 [Fe(EDTA)]− + 6 H2O + 4 H+ (Keq = 1025.1)
[Fe(EDTA)]− + 6 H2O + 4 H+ (Keq = 1025.1)
Written in this way, the equilibrium quotient shows that metal ions compete with protons for binding to EDTA. Because metal ions are extensively enveloped by EDTA, their catalytic properties are often suppressed. Finally, since complexes of EDTA4- are anionic, they tend to be highly soluble in water. For this reason, EDTA is able to dissolve deposits of metal oxides and carbonates.
Uses
Industry
In industry, EDTA is mainly used to sequester metal ions in aqueous solution. In the textile industry, it prevents metal ion impurities from modifying colours of dyed products. In the pulp and paper industry, EDTA inhibits the ability of metal ions, especially Mn2+, from catalyzing the disproportionation of hydrogen peroxide, which is used in "chlorine-free bleaching." In a similar manner, EDTA is added to some food as a preservative or stabilizer to prevent catalytic oxidative decoloration, which is catalyzed by metal ions.[8] In personal care products, it is added to cosmetics to improve their stability toward air.[9] In soft drinks containing ascorbic acid and sodium benzoate, EDTA mitigates formation of benzene (a carcinogen).[10]
The reduction of water hardness in laundry applications and the dissolution of scale in boilers both rely on EDTA and related complexants to bind Ca2+, Mg2+, as well as other metal ions. Once bound to EDTA, these metal centers tend not to form precipitates or to interfere with the action of the soaps and detergents. For similar reasons, cleaning solutions often contain EDTA.
The solubilization of ferric ions near neutral pH is accomplished using EDTA. This property is useful in agriculture including hydroponics, especially in calcareous soils. Otherwise, at near-neutral pH, iron(III) forms insoluble salts, which are less bioavailable. Aqueous [Fe(edta)]- is used for removing ("scrubbing") hydrogen sulfide from gas streams. This conversion is achieved by oxidizing the hydrogen sulfur to elemental sulfur, which is non-volatile:
- 2 [Fe(edta)]- + H2S → 2 [Fe(edta)]2− + S + 2 H+
In this application, the ferric center is reduced to its ferrous derivative, which can then be reoxidized by air. In similar manner, nitrogen oxides are removed from gas streams using [Fe(edta)]2-. The oxidizing properties of [Fe(edta)]- are also exploited in photography, where it is used to solubilize silver particles.[4]
EDTA was used in the separation of the lanthanide metals by ion-exchange chromatography. Perfected by F.H. Spedding et al. in 1954, the method relies on the steady increase in stability constant of the lanthanide EDTA complexes with atomic number. Using sulfonated polystyrene beads and copper(II) as a retaining ion, EDTA causes the lanthanides to migrate down the column of resin while separating into bands of pure lanthanide. The lanthanides elute in order of decreasing atomic number. Due to the expense of this method, relative to counter-current solvent extraction, ion-exchange is now used only to obtain the highest purities of lanthanide (typically greater than 4N, 99.99%).[citation needed]
Medicine
EDTA is used to bind metal ions in the practice of chelation therapy, e.g., for treating mercury and lead poisoning.[11] It is used in a similar manner to remove excess iron from the body. This therapy is used to treat the complication of repeated blood transfusions, as would be applied to treat thalassaemia. Alternative medical practitioners believe EDTA acts as a powerful antioxidant to prevent free radicals from injuring blood vessel walls, therefore reducing atherosclerosis.[12] The U.S. FDA approved the use of EDTA for lead poisoning[13] on July 16, 1953, under the brand name of Versenate[14], which was licensed to the pharmaceutical company Riker. It has not approved it for the treatment of atherosclerosis.[15]
Dentists and endodontists use EDTA solutions to remove inorganic debris (smear layer) and lubricate the canals in endodontics. This procedure helps prepare root canals for obturation. Furthermore, EDTA solutions with the addition of a surfactant loosen up calcifications inside a root canal and allow instrumentation (canals shaping) and facilitate apical advancement of a file in a tight/calcified root canal towards the apex. It serves as a preservative (usually to enhance the action of another preservative such as benzalkonium chloride or thiomersal) in ocular preparations and eyedrops.[16] In evaluating kidney function, the complex [Cr(edta)]- is administered intravenously and its filtration into the urine is monitored. This method is useful for evaluating glomerular filtration rate.[17]
EDTA is used extensively in the analysis of blood. It is an anticoagulant for blood samples for CBC/FBEs.
Laboratory studies also suggest that EDTA chelation may prevent collection of platelets on the lining of the vessel [such as arteries] (which can otherwise lead to formation of blood clots, which itself is associated with atheromatous plaque formation or rupture, and thereby ultimately disrupts blood flow). These ideas have so far been proven ineffective;[18] however, a major clinical study of the effects of EDTA on coronary arteries is currently (2008) proceeding.[19] EDTA played a role in the O.J. Simpson trial when the defense alleged that one of the blood samples collected from Simpson's estate was found to contain traces of the compound.[20]
EDTA is a slime dispersant, and has been found to be highly effective in reducing bacterial growth during implantation of intraocular lenses (IOLs).[21]
Laboratory applications
In the laboratory, EDTA is widely used for scavenging metal ions: In biochemistry and molecular biology, ion depletion is commonly used to deactivate metal-dependent enzymes, either as an assay for their reactivity or to suppress damage to DNA or proteins. In analytical chemistry, EDTA is used in complexometric titrations and analysis of water hardness or as a masking agent to sequester metal ions that would interfere with the analyses. EDTA finds many specialized uses in the biomedical laboratories, such as in veterinary ophthalmology as an anticollagenase to prevent the worsening of corneal ulcers in animals. In tissue culture EDTA is used as a chelating agent that binds to calcium and prevents joining of cadherins between cells, preventing clumping of cells grown in liquid suspension, or detaching adherent cells for passaging. In histopathology, EDTA can be used as a decalcifying agent making it possible to cut sections using a microtome once the tissue sample is demineralised. EDTA is also known to inhibit a range of metallopeptidases, the method of inhibition occurs via the chelation of the metal ion required for catalytic activity.[22]
Toxicity and environmental considerations
EDTA is in such widespread use that it has emerged as a persistent organic pollutant.[23] It degrades to ethylenediaminetriacetic acid, which then cyclizes to the diketopiperizide, a cumulative, persistent, organic environmental pollutant. An alternative chelating agent with fewer environmental pollution implications is EDDS.
EDTA exhibits low acute toxicity with LD50 (rat) of 2.0 – 2.2 g/kg.[4] It has been found to be both cytotoxic and weakly genotoxic in laboratory animals. Oral exposures have been noted to cause reproductive and developmental effects.[9] The same study by Lanigan[9] also found that both dermal exposure to EDTA in most cosmetic formulations and inhalation exposure to EDTA in aerosolized cosmetic formulations would produce exposure levels below those seen to be toxic in oral dosing studies.
Methods of detection and analysis
The most sensitive method of detecting and measuring EDTA in biological samples is selected-reaction-monitoring capillary-electrophoresis mass-spectrometry (abbreviation SRM-CE/MS) which has a detection limit of 7.3 ng/mL in human plasma and a quantitation limit of 15 ng/mL.[24] This method works with sample volumes as small as ~7-8 nL.[24]
EDTA has also been measured in non-alcoholic beverages using high performance liquid chromatography (HPLC) of 2.0 μg/mL.[25][26]
See also
Notes and references
- ^ Harris, D.C. "Quantitative Chemical Analysis", 7th ed., W. H. Freeman and Compagny, New York, 2007
- ^ F. Münz "Polyamino carboxylic acids to I. G. Farbenindustrie, DE 718 981, 1935; US 2 130 505, 1938.
- ^ Synthesis of EDTA
- ^ a b c J. Roger Hart "Ethylenediaminetetraacetic Acid and Related Chelating Agents" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.doi:10.1002/14356007.a10_095
- ^ Kirchner, S. Barium (Ethylenediaminetetracetato) Cobalt(III) 4-Hydrate" Inorganic Syntheses, 1957, Volume 5, pages 186-188. doi:10.1002/9780470132364.ch52
- ^ Edta - Motm
- ^ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- ^ Furia T (1964). "EDTA in Foods – A technical review". Food Technology 18 (12): 1874–1882.
- ^ a b c Lanigan RS and Yamarik TA (2002). "Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA". Int J Toxicol. 21 Suppl 2: 95–142. doi:10.1080/10915810290096522. PMID 12396676.
- ^ US Food and Drug Administration: Center for Food Safety and Applied Nutrition Questions and Answers on the Occurrence of Benzene in Soft Drinks and Other Beverages
- ^ Ruth DeBusk et al. (2002). "Ethylenediaminetetraacetic acid (EDTA)". http://www.umm.edu/altmed/articles/ethylenediaminetetraacetic-acid-000302.htm. Retrieved 2007-07-25.
- ^ "Home > Medical Reference > Complementary Medicine > EDTA overview". University of Maryland Medical Center. http://www.umm.edu/altmed/articles/ethylenediaminetetraacetic-acid-000302.htm. Retrieved 16 December 2009.
- ^ http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=26310#nlm34067-9
- ^ http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm
- ^ "Postmarket Drug Safety Information for Patients and Providers > Questions and Answers on Edetate Disodium (marketed as Endrate and generic products)". http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm113738.htm. Retrieved 16 December 2010.
- ^ See "les conservateurs en opthalmologie" Doctors Patrice Vo Tan & Yves lachkar, Librarie Médicale Théa.
- ^ Shirley, D.G., Walter, S.J. and Noormohamed, F.H. (2002). "Natriuretic effect of caffeine: assessment of segmental sodium reabsorption in humans.". Clinical Science 103 (5): 461–466. doi:10.1042/CS20020055. PMID 12401118.
- ^ Green, Saul; Wallace Sampson (December 14, 2002). "EDTA Chelation Therapy for Atherosclerosis And Degenerative Diseases: Implausibility and Paradoxical Oxidant Effects". Quackwatch. http://www.quackwatch.org/01QuackeryRelatedTopics/chelationimp.html. Retrieved 16 December 2009.
- ^ http://www.clinicaltrials.gov/ct/show/NCT00044213?order=2
- ^ Margolock, David (July 26, 1995). "F.B.I. Disputes Simpson Defense on Tainted Blood". The New York Times: pp. A12. http://query.nytimes.com/gst/fullpage.html?sec=health&res=990CE4DD1F3DF935A15754C0A963958260. Retrieved 16 December 2009.
- ^ http://jac.oxfordjournals.org/content/early/2009/01/14/jac.dkn533.full
- ^ Auld D.S "Removal and replacement of metal ions in metallopeptidases " Methods Enzymol (1995) 248, 228-242.
- ^ Zhiwen Yuan, Jeanne M. VanBriesen "The Formation of Intermediates in EDTA and NTA Biodegradation" Environmental Engineering Science 2006, volume 23, pp. 533-544. doi:10.1089/ees.2006.23.533
- ^ a b Robin L. Sheppard, and Jack Henion (1997). "Determining EDTA in Blood" (– Scholar search). Analytical Chemistry 69 (15): 477A–480A. doi:10.1021/ac971726p. PMID 9253241. http://pubs.acs.org/hotartcl/ac/97/aug/det.html. Retrieved 2007-07-25.[dead link][dead link]
- ^ S. Loyaux-Lawniczak, J. Douch, and P. Behra (1999). "Optimisation of the analytical detection of EDTA by HPLC in natural waters". Fresenius' J. Anal. Chem. 364 (8): 727–731. doi:10.1007/s002160051422. http://cat.inist.fr/?aModele=afficheN&cpsidt=1898737. Retrieved 2007-07-25.
- ^ Carolina E. Cagnassoa, Laura B. López, Viviana G. Rodríguez and Mirta E. Valencia (May 2006). "Development and validation of a method for the determination of EDTA in non-alcoholic drinks by HPLC". Journal of Food Composition and Analysis 20 (3-4): 248. doi:10.1016/j.jfca.2006.05.008.
External links
- The MEROPS online database for peptidases and their inhibitors: EDTA
- Lanigan RS, Yamarik TA (2002). "Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA". Int. J. Toxicol. 21 Suppl 2: 95–142. doi:10.1080/10915810290096522. PMID 12396676.
- EDTA: Molecule of the Month
- EDTA Determination of Total Water Hardness
- EDTA: the chelating agent under environmental scrutiny, Química Nova, Nov.-Dec., 2003 (text version)
- EDTA: the chelating agent under environmental scrutiny, Química Nova, Nov.-Dec., 2003 (PDF version)
Chelating agents / chelation therapy (V03AC, others) Iron Copper Lead BAL# • EDTA# (Dexrazoxane)Thallium Other/ungrouped Categories:- Chelating agents
- Preservatives
- Antidotes
- Acetic acids
- Amines
- World Health Organization essential medicines
- Photographic chemicals
Wikimedia Foundation. 2010.