- Organoiridium compound
-
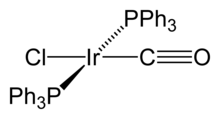
Organoiridium compounds contain iridium-carbon chemical bonds. Compounds with Ir-C bonds are found in oxidation states from 0 to V. For example, oxidation state zero is found in tetrairidium dodecacarbonyl, Ir4(CO)12, which is the most common and stable binary carbonyl of iridium.[1] In this compound, each of the iridium atoms is bonded to the other three, forming a tetrahedral cluster. Some organometallic Ir(I) compounds are notable enough to be named after their discoverers. One is Vaska's complex, IrCl(CO)[P(C6H5)3]2, which has the unusual property of reversibly binding to dioxygen molecule, O2.[2] Another one is Crabtree's catalyst, a homogeneous catalyst for hydrogenation reactions.[3] Iridium(I) complexes are both square planar, d8 complexes, with a total of 16 valence electrons, which accounts for their reactivity.[4] Many organoiridium compounds are generated from pentamethylcyclopentadienyl iridium dichloride dimer.
Uses
The dominant application of organoiridium complexes is as catalysts in the Cativa process for carbonylation of methanol to produce acetic acid[5] Iridium is competitive with rhodium for this large-scale application because of lower operating costs.
In the academic laboratories, iridium complexes are widely studied because its complexes promote C-H activation, but such reactions are not employed in any commercial process. Iridium complexes are highly active for hydrogenation both directly and via transfer hydrogenation. The asymmetric versions of these reactions are widely studied.
Iridium complexes such as Ir(mppy)3 have been examined in the context of phosphorescent organic light-emitting diode technology to increase the internal quantum efficiency from 25% to almost 100% by harvesting emission from triplet states.[6]
See also
- Category:Iridium compounds
- Other chemistries of carbon with other elements in the periodic table.
CH He CLi CBe CB CC CN CO CF Ne CNa CMg CAl CSi CP CS CCl CAr CK CCa CSc CTi CV CCr CMn CFe CCo CNi CCu CZn CGa CGe CAs CSe CBr CKr CRb CSr CY CZr CNb CMo CTc CRu CRh CPd CAg CCd CIn CSn CSb CTe CI CXe CCs CBa CHf CTa CW CRe COs CIr CPt CAu CHg CTl CPb CBi CPo CAt Rn Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo ↓ CLa CCe CPr CNd CPm CSm CEu CGd CTb CDy CHo CEr CTm CYb CLu Ac Th Pa CU Np Pu Am Cm Bk Cf Es Fm Md No Lr Chemical bonds to carbon Core organic chemistry Many uses in chemistry Academic research, but no widespread use Bond unknown / not assessed References
- ^ Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford:Butterworth-Heinemann. pp. 1113–1143, 1294. ISBN 0-7506-3365-4.
- ^ Vaska, Lauri; DiLuzio, J.W. (1961). "Carbonyl and Hydrido-Carbonyl Complexes of Iridium by Reaction with Alcohols. Hydrido Complexes by Reaction with Acid". Journal of the American Chemical Society 83 (12): 2784–2785. doi:10.1021/ja01473a054.
- ^ Crabtree, Robert H. (1979). "Iridium compounds in catalysis". Acc. Chem. Res 12 (9): 331–337. doi:10.1021/ar50141a005.
- ^ Robert H. Crabtree (2005). The Organometallic Chemistry of the Transition Metals. Wiley. ISBN 978-0-471-66256-3. http://www.wiley.com/WileyCDA/WileyTitle/productCd-0471662569.html.
- ^ Cheung, Hosea; Tanke, Robin S.; Torrence, G. Paul (2000). "Acetic acid". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a01_045.
- ^ Wang, Xiangjun; Andersson, Mats R.; Thompson, Mark E. Inganäsa, Olle (2004). "Electrophosphorescence from substituted poly(thiophene) doped with iridium or platinum complex". Thin Solid Films 468 (1-2): 226–233. Bibcode 2004TSF...468..226W. doi:10.1016/j.tsf.2004.05.095.
Categories:- Organoiridium compounds
Wikimedia Foundation. 2010.