- Helicene
-
Helicenes in organic chemistry are ortho-condensed polycyclic aromatic compounds in which benzene rings or other aromatics are angularly annulated to give helically-shaped molecules. The chemistry of helicenes has attracted continuing attention because of their unique structural, spectral, and optical features.[1]
Helicenes are notable for having chirality while lacking both asymmetric carbons and chiral centers. Helicenes' chirality results from the fact that clockwise and counterclockwise helices are non-superimposable – this is an example of axial chirality.
Contents
Background
The first [6]helicene (also called hexahelicene) was synthesized by M. S. Newman and D. Lednicer in 1956 via a scheme that closed the two central rings by Friedel-Crafts cyclization of carboxylic acid compounds.[2] Since then, several methods for synthesizing helicenes have been reported. Today, the synthesis of helicenes with different lengths and substituents is possible. The oxidative photocyclization of a stilbene-type precursor is used most often as the key step. The longest helicene, [14]helicene, was prepared in 1975 by this method.
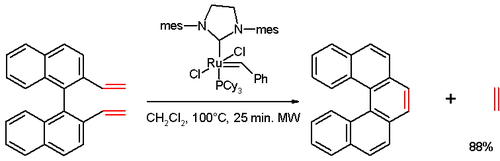
In one study,[3] [5]helicene was synthesized in an olefin metathesis reaction of a divinyl compound (prepared from 1,1'-bi-2-naphthol (BINOL) in several steps), with Grubbs' second generation catalyst:
Polyacenes are the linear 1,3-fused or meta-analogues of helicenes.
Circulenes
Closed rings consisting of benzenes are called circulenes. [5]circulene or corannulene, [6]circulene or coronene, [7]circulene[4] and [12]circulene (kekulene) have been synthesized in the laboratory. These compounds belong to a larger class of geodesic polyarenes. Whereas [5]circulene is bowl-shaped and [6]circulene is planar, [7]circulene has a unique saddle-shaped structure (compare to cones and partial cones in calixarenes).
Sulflower
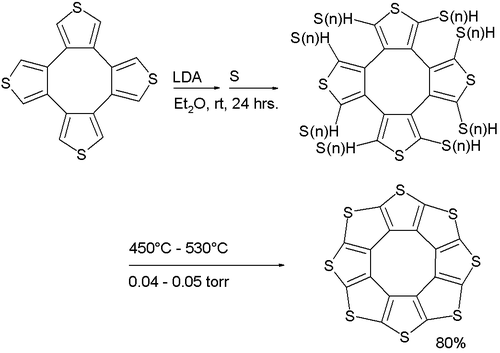
A stable octacirculene based on thiophene, not containing any hydrogen, has been reported with the name sulflower (a portmanteau of sulfur and sunflower). With molecular formula (C2S)8 the compound is considered an analogue of carbon sulfide. The molecule is flat and together with the 9-membered homologue is at a local strain energy minimum.[5]
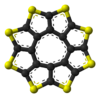

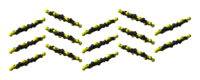
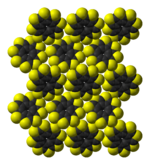
ball-and-stick model of
the sulflower moleculespace-filling model of
the sulflower moleculestacking of sulflower
molecules in the crystal structurepacking of sulflower
molecules in the crystal structureIts synthesis (a variation of the Ferrario reaction) is based on deprotonation of a tetrathiophene with lithium diisopropylamide followed by reaction with elemental sulfur to a sulfur-substituted intermediate followed by vacuum pyrolysis.
Sulflower molecules have a perfect planar structure with high symmetry (D8h). Because of their planar structure, they are predicted to store a lot of hydrogen molecules between the stacks. The conformation of the H2 molecule is calculated to be "standing up" over the five membered rings. Detailed DFT calculations have been performed on these molecules.[6]
External links
References
- ^ Diels-Alder Additions of Benzynes within Helicene Skeletons David Zhigang Wang, Thomas J. Katz, James Golen, and Arnold L. Rheingold J. Org. Chem.; 2004; 69(22) pp 7769 - 7771 DOI Graphical Abstract
- ^ Newman, M. S.; Lednicer, D. J. Am. Chem. Soc., 1956, 78, 4765-4770
- ^ Preparation of Helicenes through Olefin Metathesis Shawn K. Collins, Alain Grandbois, Martin P. Vachon, Julie Côté Angewandte Chemie International Edition Volume 45, Issue 18 , Pages 2923 - 2926 2006 Abstract
- ^ Extended systems of closed helicene. Synthesis and characterization of [7] and [7.7]-circulene Koji Yamamoto Pure & Appl. Chern., Vol. 65, No. 1, pp. 157-163, 1993. Online article
- ^ Communication Sulflower: A New Form of Carbon Sulfide Konstantin Yu. Chernichenko, Viktor V. Sumerin, Roman V. Shpanchenko, Elizabeth S. Balenkova, Valentine G. Nenajdenko Angewandte Chemie International Edition Volume 45, Issue 44 , Pages 7367 - 7370 2006 doi:10.1002/anie.200602190
- ^ Computational design of high hydrogen adsorption efficiency in molecular “Sulflower” Ayan Datta, Swapan K. Pati, J. Phys. Chem. C (Letter) 111, 4487 (2007).
2 rings 3 rings 4 rings 5 rings 6+ rings Anthanthrene · Benzo[ghi]perylene · Corannulene · Coronene · Dicoronylene · Diindenoperylene · Helicene · Heptacene · Hexacene · Kekulene · Ovalene · ZethreneCategories:- Polycyclic aromatic hydrocarbons
- Geodesic polyarenes
Wikimedia Foundation. 2010.


![[6]helicene](/pictures/enwiki/49/100px--6-helicene.svg.png)
![[7]circulene](/pictures/enwiki/49/100px--7-circulene.svg.png)