- Oxetane
-
Oxetane 
 OxetaneOther names1,3-propylene oxide
OxetaneOther names1,3-propylene oxide
1,3-epoxypropane
oxacyclobutane
trimethylene oxideIdentifiers CAS number 503-30-0 
UNII I279Q16FU6 
Jmol-3D images Image 1 - C1CCO1
Properties Molecular formula C3H6O Molar mass 58.08 g mol−1 Density 0.8930 g/cm3 Boiling point 49–50 °C
Hazards Flash point −19.0 °F (NTP, 1992)  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Oxetane, or 1,3-propylene oxide, is an heterocyclic organic compound with the molecular formula C3H6O, having a four-membered ring with three carbon atoms and one oxygen atom.
The term oxetane may also refer more generally to any organic compound containing an oxetane ring.
Contents
Production
A typical well known method of preparation is the reaction of potassium hydroxide with 3-chloropropyl acetate at 150°C[1] Yield of oxetane made this way is ca. 40%, as the synthesis can lead to a variety of by-products.
Other possible reactions to form oxetane ring is the Paternò–Büchi reaction. Also, diol cyclization can form oxetane ring.
Taxol
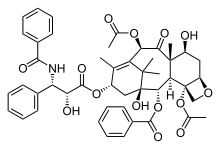 Paclitaxel with oxetane ring at right.
Paclitaxel with oxetane ring at right.
Paclitaxel (Taxol) is an example of a natural product containing an oxetane ring. Taxol has become a major point of interest among researchers due to its unusual structure and success in the involvement of cancer treatment.[2] The attached oxetane ring is an important feature that is used for the binding of microtubules in structure activity, however little is known about how the reaction is catalyzed in nature, which creates a challenge for scientists trying to synthesize the reaction.[2]
See also
- β-Propiolactone or 2-oxetanone.
- 3-Oxetanone
References
- ^ C. R. Noller (1955), "trimethylene oxide", Org. Synth. 29: 92, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV3P0835; Coll. Vol. 3: 835
- ^ a b Willenbring, Dan, and Dean J. Tantillo.. "Mechanistic possibilities for oxetane formation in the biosynthesis of Taxol’s D ring." Russian Journal of General Chemistry 78.4 (Apr. 2008): 7237–31. Advanced Placement Source. EBSCO. [Library name], [City], [State abbreviation]. 22 Apr. 2009 <http://search.ebscohost.com/login.aspx?direct=true&db=aqh&AN=32154883&site=ehost-live>
Categories:- Oxetanes
Wikimedia Foundation. 2010.
