- Cytochrome b559
-
Cytochrome b559, alpha (gene psbE) and beta (gene psbF)subunits 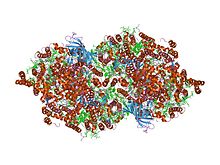
Structure of Photosystem II from Thermosynechococcus elongatus.[1] Identifiers Symbol Cytochrom_B559 Pfam PF00283 InterPro IPR013081 PROSITE PDOC00464 OPM family 2 OPM protein 2axt Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Lumenal portion of Cytochrome b559, alpha (gene psbE) subunit 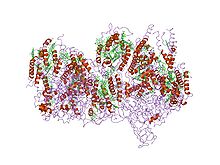
Structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus.[2] Identifiers Symbol Cytochrom_B559a Pfam PF00284 InterPro IPR013082 PROSITE PDOC00464 OPM family 2 OPM protein 2axt Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Cytochrome b559 is an important component of Photosystem II.
PSII is a multisubunit protein-pigment complex containing polypeptides both intrinsic and extrinsic to the photosynthetic membrane[3][4]. Within the core of the complex, the chlorophyll and beta-carotene pigments are mainly bound to the antenna proteins CP43 (PsbC) and CP47 (PsbB), which pass the excitation energy on to chlorophylls in the reaction centre proteins D1 (Qb, PsbA) and D2 (Qa, PsbD) that bind all the redox-active cofactors involved in the energy conversion process. The PSII oxygen-evolving complex (OEC) provides electrons to re-reduce the PSII reaction center and oxidizes 2 water molecules to recover its reduced initial state. It consists of OEE1 (PsbO), OEE2 (PsbP) and OEE3 (PsbQ). The remaining subunits in PSII are of low molecular weight (less than 10 kDa), and are involved in PSII assembly, stabilisation, dimerisation, and photo-protection[5].
Cytochrome b559, which forms part of the reaction centre core of PSII is a heterodimer composed of one alpha subunit (PsbE), one beta (PsbF) subunit, and a heme cofactor. Two histidine residues from each subunit coordinate the haem. Although cytochrome b559 is a redox-active protein, it is unlikely to be involved in the primary electron transport in PSII due to its very slow photo-oxidation and photo-reduction kinetics. Instead, cytochrome b559 could participate in a secondary electron transport pathway that helps protect PSII from photo-damage. Cytochrome b559 is essential for PSII assembly[6].
This domain occurs in both the alpha and beta subunits of cytochrome B559. In the alpha sbunit it occurs together with a lumenal domain (IPR013082), while in the beta subunit it occurs on its own.
Cytochrome b559 can exist in three forms, each with a characteristic redox potential, These forms are very low potential (VLP), ≤ zero mV; low potential (LP) at 60 mV; and high potential (HP) at 370 mV. There is also an intermediate potential (IP) form that has a redox potential at pH 6.5-7.0 that ranges from 170 to 240 mV. In oxygen evolving reaction centers, more than half of the cyt b559 is in the HP form. In manganese depleted non oxygen evolving photosystem II reaction centers, cyt b559 is usually in the LP form[7].
References
- ^ Loll B, Kern J, Saenger W, Zouni A, Biesiadka J (December 2005). "Towards complete cofactor arrangement in the 3.0 A resolution structure of photosystem II". Nature 438 (7070): 1040–4. doi:10.1038/nature04224. PMID 16355230.
- ^ Kamiya N, Shen JR (January 2003). "Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7-A resolution". Proc. Natl. Acad. Sci. U.S.A. 100 (1): 98–103. doi:10.1073/pnas.0135651100. PMC 140893. PMID 12518057. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=140893.
- ^ Kamiya N, Shen JR (2003). "Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7-A resolution". Proc. Natl. Acad. Sci. U.S.A. 100 (1): 98–103. doi:10.1073/pnas.0135651100. PMC 140893. PMID 12518057. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=140893.
- ^ Blankenship RE, Raymond J (2004). "The evolutionary development of the protein complement of photosystem 2". Biochim. Biophys. Acta 1655 (1-3): 133–139. doi:10.1016/j.bbabio.2003.10.015. PMID 15100025.
- ^ Schroder WP, Shi LX (2004). "The low molecular mass subunits of the photosynthetic supracomplex, photosystem II". Biochim. Biophys. Acta 1608 (2-3): 75–96. doi:10.1016/j.bbabio.2003.12.004. PMID 14871485.
- ^ Burda K, Kruk J, Borgstadt R, Stanek J, StrzaBka K, Schmid GH, Kruse O (2003). "Mössbauer studies of the non-heme iron and cytochrome b559 in a Chlamydomonas reinhardtii PSI- mutant and their interactions with alpha-tocopherol quinone". FEBS Lett. 535 (1-3): 159–165. doi:10.1016/S0014-5793(02)03895-4. PMID 12560096.
- ^ Mizusawa N, Yamashita T, Miyao M (1999). "Restoration of the high-potential form of cytochrome b559 of photosystem II occurs via a two-step mechanism under illumination in the presence of manganese ions". BBA 1410 (3): 273–286. doi:10.1016/S0005-2728(99)00005-5. PMID 10082793.
Categories:- Photosynthesis
- Protein domains
- Protein families
- Single-pass transmembrane proteins
Wikimedia Foundation. 2010.
