- Outer membrane phospholipase A1
-
Phospholipase A1 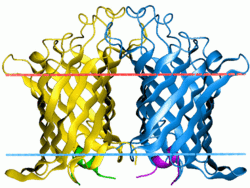
Identifiers Symbol PLA1 Pfam PF02253 InterPro IPR003187 SCOP 1qd6 OPM family 29 OPM protein 1qd6 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Outer membrane phospholipase A1 (OMPLA) is an acyl hydrolase with a broad substrate specificity (EC:3.1.1.32.) from the bacterial outer membrane. It has been proposed that Ser164 is the active site of the protein (UniProt P00631) [1]
This integral membrane phospholipase was found in many Gram-negative bacteria and has a broad substrate specificity EC 3.1.1.32. The role of OMPLA has been most thoroughly studied in Escherichia coli, where it participates in the secretion of bacteriocins. Bacteriocin release is triggered by a lysis protein (bacteriocin release protein or BRP), followed by a phospholipase dependent accumulation of lysophospholipids and free fatty acids in the outer membrane[2]. The reaction products enhance the permeability of the outer membrane, which allows the semispecific secretion of bacteriocins. One speculative function of OMPLA is related to organic solvent tolerance in bacteria.
Structurally, it consists of a 12-stranded antiparallel beta-barrel with a convex and a flat side. The active site residues are exposed on the exterior of the flat face of the beta-barrel. The activity of the enzyme is regulated by reversible dimerisation. Dimer interactions occur exclusively in the membrane-embedded parts of the flat side of the beta-barrel, with polar residues embedded in an apolar environment forming the key interactions. The active site His and Ser residues are located at the exterior of the beta-barrel, at the outer leaflet side of the membrane. This location indicates that under normal conditions the substrate and the active site are physically separated, since in E. coli phospholipids are exclusively located in the inner leaflet of the outer membrane. [3].
References
- ^ * Inactivation of Escherichia coli outer-membrane phospholipase A by the affinity label hexadecanesulfonyl fluoride. Evidence for an active-site serine. Horrevoets AJ, Verheij HM, de Haas GH; Eur J Biochem 1991;198:247-253. PubMed
- ^ Kalk KH, Dijkstra BW, Timmins PA, Snijder HJ (2003). "Detergent organisation in crystals of monomeric outer membrane phospholipase A". J. Struct. Biol. 141 (2): 122–131. doi:10.1016/S1047-8477(02)00579-8. PMID 12615538.
- ^ IPR003187

This membrane protein-related article is a stub. You can help Wikipedia by expanding it.
