- Mesenchymal stem cell
-
Mesenchymal stem cell 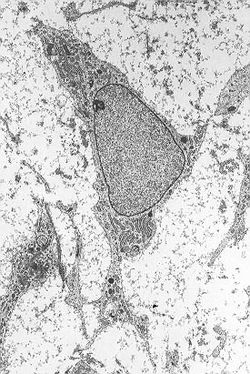
Mesenchymal stem cell showing typical ultrastructural morphology. Latin cellula mesenchymatica precursoria Code TH H2.00.01.0.00008 Mesenchymal stem cells, or MSCs, are multipotent stem cells that can differentiate into a variety of cell types,[1] including: osteoblasts (bone cells), chondrocytes (cartilage cells) and adipocytes (fat cells). This has been shown in ex vivo cultures and in vitro or in vivo.
Contents
Definition
While the terms Mesenchymal Stem Cell and Marrow Stromal Cell have been used interchangeably, neither term is sufficiently descriptive as discussed below:
- Mesenchyme is embryonic connective tissue that is derived from the mesoderm and that differentiates into hematopoietic and connective tissue, whereas MSCs do not differentiate into hematopoietic cells.[citation needed]
- Stromal cells are connective tissue cells that form the supportive structure in which the functional cells of the tissue reside. While this is an accurate description for one function of MSCs, the term fails to convey the relatively recently-discovered roles of MSCs in the repair of tissue.[citation needed]
- Because the cells, called MSCs by many labs today, can encompass multipotent cells derived from other non-marrow tissues, such as umbilical cord blood, adipose tissue, adult muscle or the dental pulp of deciduous baby teeth, yet do not have the capacity to reconstitute an entire organ, the term Multipotent Stromal Cell has been proposed as a better replacement.
The youngest, most primitive MSC’s can be obtained from the umbilical cord tissue, namely Wharton’s jelly and the umbilical cord blood. However the MSC’s are found in much higher concentration in the Wharton’s jelly compared to the umbilical cord blood, which is a rich source of hematopoeitic stem cells. The umbilical cord is easily obtained after the birth of the newborn , is normally thrown away and poses no risk for collection. The umbilical cord MSCs have more primitive properties than other adult MSCs obtained later in life, which might make them a useful source of MSCs for clinical applications.
An extremely rich source for mesenchymal stem cells is the developing tooth bud of the mandibular third molar. While considered multipotent, they may prove to be pluripotent. The stem cells eventually form enamel, dentin, blood vessels, dental pulp, nervous tissues, including a minimum of 29 different unique end organs. Because of extreme ease in collection at 8–10 years of age before calcification and minimal to no morbidity they will probably constitute a major source for personal banking, research and multiple therapies. These stem cells have been shown capable of producing hepatocytes. Additionally, amniotic fluid has been shown to be a very rich source of stem cells. As many as 1 in 100 cells collected from and genetic amniocentesis has been shown to be a pluripotent mesenchymal stem cell.[citation needed]
Adipose tissue is one of the richest sources of MSC’s. When compared to bone marrow, there is more than 500 times more stem cells in 1 gram of fat when compared to 1 gram of aspirated bone marrow. Adipose stem cells are currently actively being researched in clinical trials for treatment in a variety of diseases.
History
In 1924, Russian-born morphologist Alexander A. Maximow used extensive histological findings to identify a singular type of precursor cell within mesenchyme that develops into different types of blood cells.[2]
Scientists Ernest A. McCulloch and James E. Till first revealed the clonal nature of marrow cells in the 1960s.[3][4] An ex vivo assay for examining the clonogenic potential of multipotent marrow cells was later reported in the 1970s by Friedenstein and colleagues.[5][6] In this assay system, stromal cells were referred to as colony-forming unit-fibroblasts (CFU-f).
Subsequent experimentation revealed the plasticity of marrow cells and how their fate could be determined by environmental cues. Culturing marrow stromal cells in the presence of osteogenic stimuli such as ascorbic acid, inorganic phosphate, and dexamethasone could promote their differentiation into osteoblasts. In contrast, the addition of transforming growth factor-beta (TGF-b) could induce chondrogenic markers.[citation needed]
Characteristics
Morphology
Mesenchymal stem cells are characterized morphologically by a small cell body with a few cell processes that are long and thin. The cell body contains a large, round nucleus with a prominent nucleolus, which is surrounded by finely dispersed chromatin particles, giving the nucleus a clear appearance. The remainder of the cell body contains a small amount of Golgi apparatus, rough endoplasmic reticulum, mitochondria, and polyribosomes. The cells, which are long and thin, are widely dispersed and the adjacent extracellular matrix is populated by a few reticular fibrils but is devoid of the other types of collagen fibrils.[7][8]
Detection
There is no test that can be performed on a single cell to determine whether that cell is an MSC. There are surface antigens that can be used to isolate a population of cells that have similar self-renewal and differentiation capacities, yet MSCs, as a population, typically do not all express the proposed markers; and it is not certain which ones must be expressed in order for that cell to be classified as an MSC. It may be that the therapeutic properties attributed to MSCs result from the interaction between the different cells that make up an MSC culture, suggesting that there is no one cell that has all the properties.[citation needed]
Differentiation capacity
MSCs have a large capacity for self-renewal while maintaining their multipotency. Beyond that, there is little that can be definitively said. The standard test to confirm multipotency is differentiation of the cells into osteoblasts, adipocytes, and chondrocytes as well as myocytes and neurons. MSCs have been seen to even differentiate into neuron-like cells,[9] but there is lingering doubt whether the MSC-derived neurons are functional.[10] The degree to which the culture will differentiate varies among individuals and how differentiation is induced, e.g., chemical vs. mechanical;[11] and it is not clear whether this variation is due to a different amount of "true" progenitor cells in the culture or variable differentiation capacities of individuals' progenitors. The capacity of cells to proliferate and differentiate is known to decrease with the age of the donor, as well as the time in culture. Likewise, whether this is due to a decrease in the number of MSCs or a change to the existing MSCs is not known.[citation needed]
Immunomodulatory effects
Numerous studies have demonstrated that human MSC avoid allorecognition, interfere with dendritic cell and T-cell function, and generate a local immunosuppressive microenvironment by secreting cytokines.[12] It has also been shown that the immunomodulatory function of human MSC is enhanced when the cells are exposed to an inflammatory environment characterised by the presence of elevated local interferon-gamma levels.[13] Other studies contradict some of these findings, reflecting both the highly heterogeneous nature of MSC isolates and the considerable differences between isolates generated by the many different methods under development.[14]
Culturing
The majority of modern culture techniques still take a CFU-f approach, where raw unpurified bone marrow or ficoll-purified bone marrow Mononuclear cell are plated directly into cell culture plates or flasks. Mesenchymal stem cells, but not red blood cells or haematopoetic progenitors, are adherent to tissue culture plastic within 24 to 48 hours. However, at least one publication has identified a population of non-adherent MSCs that are not obtained by the direct-plating technique.[15]
Other flow cytometry-based methods allow the sorting of bone marrow cells for specific surface markers, such as STRO-1.[16] STRO-1+ cells are generally more homogenous, and have higher rates of adherence and higher rates of proliferation, but the exact differences between STRO-1+ cells and MSCs are not clear.[17]
Methods of immunodepletion using such techniques as MACS have also been used in the negative selection of MSCs.[citation needed]
Clinical use
The mesenchymal stem cells can be activated and mobilized if needed. However, the efficiency is very low. For instance, damage to muscles heals very slowly. However, if there were a method of activating the mesenchymal stem cells, then such wounds would heal much faster.[citation needed]
Many of the early clinical successes using intravenous transplantation have come in systemic diseases like graft versus host disease and sepsis. However, it is becoming more accepted that diseases involving peripheral tissues, such as inflammatory bowel disease, may be better treated with methods that increase the local concentration of cells.[18] Direct injection or placement of cells into a site in need of repair may be the preferred method of treatment, as vascular delivery suffers from a "pulmonary first pass effect" where intravenous injected cells are sequestered in the lungs.[19] Clinical case reports in orthopedic applications have been published, though the number of patients treated is small and these methods still lack rigorous study demonstrating effectiveness. Wakitani has published a small case series of nine defects in five knees involving surgical transplantation of mesenchymal stem cells with coverage of the treated chondral defects.[20]
See also
References
- ^ Nardi, N. Beyer; da Silva Meirelles, L. (2006). "Mesenchymal Stem Cells: Isolation, In Vitro Expansion and Characterization". In Wobus, Anna M.; Boheler, Kenneth. Stem Cells. Handbook of experimental pharmacology. 174. pp. 249–82. doi:10.1007/3-540-31265-X_11. ISBN 978-3-540-77854-7. http://books.google.com/?id=aGyqLIoP1kUC&pg=PA248.
- ^ Sell, Stewart (Stem cell handbook). Humana Press. p. 143.
- ^ Becker, A. J.; McCulloch, E. A.; Till, J. E. (1963). "Cytological Demonstration of the Clonal Nature of Spleen Colonies Derived from Transplanted Mouse Marrow Cells". Nature 197 (4866): 452–4. doi:10.1038/197452a0. PMID 13970094.
- ^ Siminovitch, L.; McCulloch, E. A.; Till, J. E. (1963). "The distribution of colony-forming cells among spleen colonies". Journal of Cellular and Comparative Physiology 62 (3): 327–36. doi:10.1002/jcp.1030620313. PMID 14086156.
- ^ Friedenstein, AJ; Deriglasova, UF; Kulagina, NN; Panasuk, AF; Rudakowa, SF; Luriá, EA; Ruadkow, IA (1974). "Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method". Experimental hematology 2 (2): 83–92. PMID 4455512.
- ^ Friedenstein, AJ; Gorskaja, JF; Kulagina, NN (1976). "Fibroblast precursors in normal and irradiated mouse hematopoietic organs". Experimental hematology 4 (5): 267–74. PMID 976387.
- ^ Netter, Frank H. (1987). Musculoskeletal system: anatomy, physiology, and metabolic disorders. Summit, New Jersey: Ciba-Geigy Corporation. p. 134. ISBN 0-914168-88-6.
- ^ Brighton, Carl T.; Hunt, Robert M. (1991). "Early histological and ultrastructural changes in medullary fracture callus". The Journal of Bone and Joint Surgery 73 (6): 832–47. PMID 2071617. http://www.ejbjs.org/cgi/reprint/73/6/832.
- ^ Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD et al. (2002). "Pluripotency of mesenchymal stem cells derived from adult marrow". Nature 418 (6893): 41–49. doi:10.1038/nature00870. PMID 12077603.
- ^ Franco Lambert AP, Fraga Zandonai A, Bonatto D, Cantarelli Machado D, Pêgas Henriques JA (2009). "Differentiation of human adipose-derived adult stem cells into neuronal tissue: Does it work?". Differentiation 77 (3): 221–8. doi:10.1016/j.diff.2008.10.016. PMID 19272520.
- ^ Engler, A; Sen, S; Sweeney, H; Discher, D (2006). "Matrix Elasticity Directs Stem Cell Lineage Specification". Cell 126 (4): 677–89. doi:10.1016/j.cell.2006.06.044. PMID 16923388.
- ^ Ryan, Jennifer M; Barry, Frank P; Murphy, J Mary; Mahon, Bernard P (2005). "Mesenchymal stem cells avoid allogeneic rejection". Journal of Inflammation 2: 8. doi:10.1186/1476-9255-2-8. PMC 1215510. PMID 16045800. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1215510.
- ^ Ryan, J. M.; Barry, F.; Murphy, J. M.; Mahon, B. P. (2007). "Interferon-γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells". Clinical & Experimental Immunology 149 (2): 353–63. doi:10.1111/j.1365-2249.2007.03422.x. PMC 1941956. PMID 17521318. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1941956.
- ^ Phinney, Donald G.; Prockop, Darwin J. (2007). "Concise Review: Mesenchymal Stem/Multipotent Stromal Cells: The State of Transdifferentiation and Modes of Tissue Repair-Current Views". Stem Cells 25 (11): 2896–902. doi:10.1634/stemcells.2007-0637. PMID 17901396.
- ^ Wan, Chao; He, Qiling; McCaigue, Mervyn; Marsh, David; Li, Gang (2006). "Nonadherent cell population of human marrow culture is a complementary source of mesenchymal stem cells (MSCs)". Journal of Orthopaedic Research 24 (1): 21–8. doi:10.1002/jor.20023. PMID 16419965.
- ^ Gronthos, S; Graves, SE; Ohta, S; Simmons, PJ (1994). "The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors". Blood 84 (12): 4164–73. PMID 7994030. http://www.bloodjournal.org/cgi/pmidlookup?view=long&pmid=7994030.
- ^ Oyajobi, Babatunde O.; Lomri, Abderrahim; Hott, Monique; Marie, Pierre J. (1999). "Isolation and Characterization of Human Clonogenic Osteoblast Progenitors Immunoselected from Fetal Bone Marrow Stroma Using STRO-1 Monoclonal Antibody". Journal of Bone and Mineral Research 14 (3): 351–61. doi:10.1359/jbmr.1999.14.3.351. PMID 10027900.
- ^ Manieri, Nicholas A; Stappenbeck, Thaddeus S (2011). "Mesenchymal stem cell therapy of intestinal disease: are their effects systemic or localized?". Current Opinion in Gastroenterology 27 (2): 119–24. doi:10.1097/MOG.0b013e3283423f20. PMID 21150589.
- ^ Fischer, Uwe M.; Harting, Matthew T.; Jimenez, Fernando; Monzon-Posadas, Werner O.; Xue, Hasen; Savitz, Sean I.; Laine, Glen A.; Cox, Charles S. (2009). "Pulmonary Passage is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect". Stem Cells and Development 18 (5): 683–92. doi:10.1089/scd.2008.0253. PMID 19099374.
- ^ Wakitani, Shigeyuki; Nawata, Masashi; Tensho, Keiji; Okabe, Takahiro; MacHida, Hiroko; Ohgushi, Hajime (2007). "Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees". Journal of Tissue Engineering and Regenerative Medicine 1 (1): 74–9. doi:10.1002/term.8. PMID 18038395.
Cellular differentiation: Stem cells / progenitor cell Sources/types Cell potency Totipotent (Zygote, Spore, Morula) · Pluripotent (Embryonic stem cell, Callus) · Multipotent (Progenitor cell: Endothelial stem cell, Hematopoietic stem cell, Mesenchymal stem cell, Neural stem cell) · Unipotent (Precursor cell)Related articles Stem cell treatments · Stem cell controversy · Stem cell line · Stem cell laws · Stem cell laws and policy in the United StatesHuman cell types / list derived primarily from mesoderm Paraxial muscle: Myoblast → Myocyte · Myosatellite cell · Tendon cell · Cardiac muscle cell
adipose: Lipoblast → AdipocyteDigestive systemIntermediate Urinary system (RSC)Angioblast → Endothelial cell · Mesangial cell (Intraglomerular, Extraglomerular) · Juxtaglomerular cell · Macula densa cell
Stromal cell → Interstitial cell → Telocytes
Simple epithelial cell → Podocyte · Kidney proximal tubule brush border cellLateral plate/
hemangioblastsee lymphocytessee myeloid cellsCategories:- Stem cells
Wikimedia Foundation. 2010.
