- Phthalocyanine
-
Phthalocyanine[1]  Other namesPhthalocyanin
Other namesPhthalocyaninIdentifiers CAS number 574-93-6 
PubChem 5282330 Jmol-3D images {{#if:C1=CC=C2C(=C1)C3=NC4=C5C=CC=CC5=C(N4)N=C6C7=CC=CC=C7C(=N6)N=C8C9=CC=CC= C9C(=N8)N=C2N3|Image 1
- C1=CC=C2C(=C1)C3=NC4=C5C=CC=CC5=C(N4)N=C6C7=CC=CC=C7C(=N6)N=C8C9=CC=CC= C9C(=N8)N=C2N3
Properties Molecular formula C32H18N8 Molar mass 514.54 g mol−1 Hazards S-phrases S22 S24/25  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Phthalocyanine is an intensely blue-green coloured macrocyclic compound that is widely used in dyeing.[2][3][4] Phthalocyanines form coordination complexes with most elements of the periodic table. These complexes are also intensely colored and also are used as dyes or pigments.
Contents
Properties
Unsubstituted phthalocyanine, abbreviated H2Pc, and many of its complexes have very low solubility in organic solvents. Benzene at 40 °C dissolves less than a milligram of H2Pc or CuPc per litre. H2Pc or CuPc dissolve easily in sulfuric acid due to the protonation of the nitrogen atoms bridging the pyrrole rings. Many phthalocyanine compounds are thermally very stable, do not melt but can be sublimed, CuPc sublimes at >500 °C under inert gases (nitrogen, CO2). Substituted phthalocyanine complexes often have much higher solubility. They are less thermally stable and often can not be sublimed. Unsubstituted phthalocyanines strongly absorb light between 600 to 700 nm, thus these materials are blue or green. Substitution can shift the absorption towards longer wavelengths, changing the color from pure blue to green to colorless (when the absorption is in the near infrared.
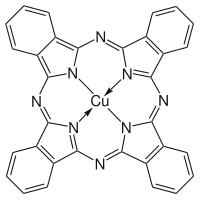
Phthalocyanines are structurally related to other macrocyclic pigments, especially the porphyrins. Both feature four pyrrole-like subunits linked to form a 16-membered ring. The pyrrole-like rings within H2Pc are closely related to isoindole. Both porphyrins and phthalocyanines function as planar tetradentate dianionic ligands that bind metals through four inwardly projecting nitrogen centers. Such complexes are formally derivatives of Pc2-, the conjugate base of H2Pc.
Many derivatives of the parent phthalocyanine are known, where either carbon atoms of the macrocycle are exchanged for nitrogen atoms or where the hydrogen atoms of the ring are substituted by functional groups like halogens, hydroxy, amino, alkyl, aryl, thiol, alkoxy, nitro, etc. groups.
History
An unidentified blue compound, which is now known to be metal-free phthalocyanine, was first reported in 1907.[5] In 1927, Swiss researchers accidentally synthesized copper phthalocyanine, copper naphthalocyanine, and copper octamethylphthalocyanine in an attempted conversion of o-dibromobenzene into phthalonitrile. They remarked on the enormous stability of these complexes but did not further characterize these blue complexes.[6] In the same year, copper phthalocyanine was also discovered at Scottish Dyes, Ltd., Grangemouth, Scotland (later ICI). ColorantHistory.org hosts an old documentary about the discovery of the pigment.
Synthesis
Phthalocyanine forms upon heating phthalic acid derivatives that contain nitrogen functional groups. Classical precursors are phthalonitrile and diiminoisoindole. In the presence of urea, the heating of phthalanhydride is a useful route to H2Pc. These reactions are more efficient in the presence of metal salts. Other precursors include , o-cyanobenzamide, , and phthalimide. Several of these starting materials are shown in the figure below (right side). Using such methods, approximately 57000 tons of various phthalocyanines were produced in 1985.[7]
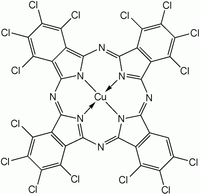
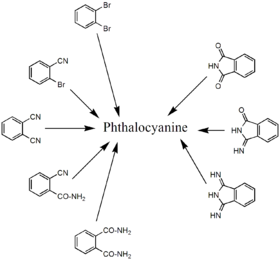
Halogenated and sulfonated derivatives of copper phthalocyanines are commercially important. Such compounds are prepared by treating CuPc with chlorine, bromine or oleum.
Applications
Approximately 25% of all artificial organic pigments are phthalocyanine derivatives.[7] Copper phthalocyanine (CuPc) dyes are produced by introducing solubilizing groups, such as one or more sulfonic acid functions. These dyes find extensive use in various areas of textile dyeing (Direct dyes for cotton), for spin dyeing and in the paper industry. Direct blue 86 is the sodium salt of CPC-sulfonic acid whereas direct blue 199 is the quaternary ammonium salt of the CPC-sulfonic acid. The quaternary ammonium salts of these sulfonic acids used as solvent dyes because of their solubility in organic solvents, e.g. Solvent Blue 38 and Solvent Blue 48. The dye derived from cobalt phthalocyanine and an amine is Phthalogen Dye IBN. 1,3-Diiminoisoindolene, the intermediate formed during phthalocyanine manufacture, used in combination with a copper salt affords the dye GK 161.
All major artists' pigment manufacturers produce variants of copper phthalocyanine, designated color index PB15 (blue) and color indexes PG7 and PG36 (green).
Phthalocyanine is also commonly used as a dye in the manufacture of high-speed CD-R media. Most brands of CD-R use this dye except Taiyo Yuden and Verbatim DataLife (which use cyanine and azo dyes, respectively).
Niche applications
Metal phthalocyanines have long been examined as catalysts for redox reactions. Areas of interest are the oxygen reduction reaction and the sweetening of gas streams by removal of hydrogen sulfide.
Phthalocyanine compounds have been investigated as donor materials in molecular electronics, e.g. organic field-effect transistors.[8]
Toxicity and Hazards
There is evidence that exposure to phthalocyanines can cause serious birth defects in developing embryos.[9]
Related compounds
Phthalocyanines are closely related to other tetrapyrrole macrocyles including porphyrins and porphyrazines.
References
- ^ Phthalocyanine at Sigma-Aldrich
- ^ The Phthalocyanines - Vols 1-4 Edited by C. C.Leznoff and A.B.P.Lever, Wiley 1986-1993.
- ^ "Phthalocyanine Materials - Synthesis, Structure and Function", Neil B. McKeown, Cambridge University Press 1998
- ^ The Porphyrin Handbook, Vols. 15-20; Karl Kadish, Kevin M. Smith, Roger Guilard (eds); Academic Press 2003.
- ^ A.Braun, J.Tcherniac, Berichte der Deutschen Chemischen Gesellschaft, 1907, volume 40, 2709.
- ^ H. de Diesbach, E. von der Weid, Helevtica Chimica Acta, 1927, volume 10, 886.
- ^ a b Gerd Löbbert "Phthalocyanines" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a20_213.
- ^ Illustrative paper: G. Chaidogiannos et al., Low voltage operating OFETs based on solution-processed metal phthalocyanines, Applied Physics A: Materials Science & Processing, 2009, 96, p.763.
- ^ "Sulphonated phthalocyanine induced caudal malformative syndrome in the chick embryo." (Abstract), Sandor S, Prelipceanu O, and Checiu I., U.S. National Library of Medicine National Institutes of Health
External links
Categories:- Phthalocyanines
- Macrocycles
Wikimedia Foundation. 2010.

