- Pericyclic reaction
-
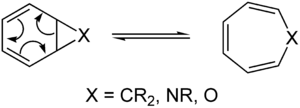
In organic chemistry, a pericyclic reaction is a type of organic reaction wherein the transition state of the molecule has a cyclic geometry, and the reaction progresses in a concerted fashion. Pericyclic reactions are usually rearrangement reactions. The major classes of pericyclic reactions are:
Name Bond changes Sigma Pi Electrocyclic reaction + 1 -1 Cycloaddition +2 -2 Sigmatropic reaction 0 0 Group transfer reaction + 1 -1 Cheletropic reaction + 2 - 2 Dyotropic reaction 0 0 In general, these are considered to be equilibrium processes, although it is possible to push the reaction in one direction by designing a reaction by which the product is at a significantly lower energy level; this is due to a unimolecular interpretation of Le Chatelier's principle. Pericyclic reactions often have related stepwise radical processes associated with them. Some pericyclic reactions, such as the [2+2] cycloaddition, are 'controversial' because their mechanism is not definitively known to be concerted (or may depend on the reactive system). Pericyclic reactions also often have metal-catalyzed analogs, although usually these are also not technically pericyclic, since they proceed via metal-stabilized intermediates, and therefore are not concerted.
A large photoinduced hydrogen sigmatropic shift was utilized in a corrin synthesis performed by Albert Eschenmoser containing a 16π system.[1]
Due to the principle of microscopic reversibility, there is a parallel set of "retro" pericyclic reactions, which perform the reverse reaction.
Pericyclic reactions in biochemistry
Pericyclic reactions also occur in several biological processes.
- Claisen rearrangement of chorismate to prephenate in almost all prototrophic organisms.
- [1,5]-sigmatropic shift in the transformation of precorrin-8x to hydrogenobyrinic acid
- non-enzymatic, photochemical electrocyclic ring opening and a (1,7) sigmatropic hydride shift in vitamin D synthesis.
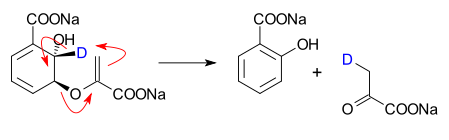
- a conversion of Isochorismate into salicylate and Pyruvate in a catalyzed, true pericyclic reaction [2][3].
See also
- Woodward-Hoffmann rules
References
- ^ A New Type of Corrin Synthesis. Yasuji Yamada, D. Miljkovic, P. Wehrli, B. Golding, P. Loliger, R. Keese, K. Miiller, and A. Eschenmoser. Angew. Chem. Int. Edit. 1969, 8(5),343-348.
- ^ Isochorismate Pyruvate Lyase: A Pericyclic Reaction Mechanism? Michael S. DeClue, Kim K. Baldridge, Dominik E. Künzler, Peter Kast, and Donald Hilvert J. Am. Chem. Soc.; 2005; 127(43) pp 15002 - 15003; (Communication) DOI: 10.1021/ja055871t Abstract
- ^ In this experiment isochorismate is deuterated in one specific position and subjected to the lyase. Two key observations rule out other reaction mechanisms, ionic or base promoted. From the kinetic isotope effect (value 2.34) it can be inferred that carbon to hydrogen bond breaking occurs in the transition state of the rate determining step. NMR spectroscopy shows that the deuterium atom is transferred exclusively to the pyruvate molecule.
Categories:- Rearrangement reactions
- Reaction mechanisms
- Pericyclic reactions
Wikimedia Foundation. 2010.