- Dimethyltryptamine
-
Dimethyltryptamine 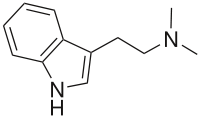
Systematic (IUPAC) name 2-(1H-indol-3-yl)-N,N-dimethylethanamine Clinical data Pregnancy cat. ? Legal status Prohibited (S9) (AU) Schedule III (CA) CD Lic (UK) Schedule I (US) Routes Oral (with an MAOI), Insufflated, Rectal, Smoked (or vaporized), IM, IV Identifiers CAS number 61-50-7 
ATC code None PubChem CID 6089 IUPHAR ligand 141 DrugBank DB01488 ChemSpider 5864 
UNII WUB601BHAA 
KEGG C08302 
ChEBI CHEBI:28969 
ChEMBL CHEMBL12420 
Chemical data Formula C12H16N2 Mol. mass 188.269 g/mol SMILES eMolecules & PubChem Physical data Density 1.099g/ml g/cm³ Melt. point 40 °C (104 °F) Boiling point 160 °C (320 °F) @ 0.6 Torr[1] also reported as 80 - 135 °C @ 0.03 Torr [2]  (what is this?) (verify)
(what is this?) (verify)N,N-Dimethyltryptamine (DMT) is a naturally occurring psychedelic compound of the tryptamine family. DMT is found in several plants,[3] and also in trace amounts in humans and other mammals, where it is originally derived from the essential amino acid tryptophan, and ultimately produced by the enzyme INMT during normal metabolism.[4] The natural function of its widespread presence remains undetermined. Structurally, DMT is analogous to the neurotransmitter serotonin (5-HT), the hormone melatonin, and other psychedelic tryptamines, such as 5-MeO-DMT, bufotenin, and psilocin (the active metabolite of psilocybin).
In some cultures DMT is ingested as a psychedelic drug (in either extracted or synthesized forms).[5] When DMT is inhaled or consumed, depending on the dose, its subjective effects can range from short-lived milder psychedelic states to powerful immersive experiences, which include a total loss of connection to conventional reality, which may be so extreme that it becomes ineffable.[6] DMT is also the primary psychoactive in ayahuasca, an Amazonian Amerindian brew employed for divinatory and healing purposes. Pharmacologically, ayahuasca combines DMT with an MAOI, an enzyme inhibitor that allows DMT to be orally active.[7]
Contents
History
DMT was first synthesized in 1931 by Canadian chemist Richard Manske (1901–1977).[8][9] Its discovery as a natural product is generally credited to Brazilian chemist and microbiologist Oswaldo Gonçalves de Lima (1908–1989) who, in 1946, isolated an alkaloid he named nigerina (nigerine) from the root bark of jurema preta, that is, Mimosa tenuiflora.[9][10][11] However, in a careful review of the case Jonathan Ott shows that the empirical formula for nigerine determined by Gonçalves de Lima, which notably contains an atom of oxygen, can only match a partial, "impure" or "contaminated" form of DMT.[12] It was only in 1959, when Gonçalves de Lima provided American chemists a sample of Mimosa tenuiflora roots, that DMT was unequivocally identified in this plant material.[12][13] Less ambiguous is the case of isolation and formal identification of DMT in 1955 in seeds and pods of Anadenanthera peregrina by a team of American chemists led by Evan Horning (1916–1993).[12][14] Since 1955 DMT has been found in a host of organisms: in at least 50 plant species belonging to 10 families,[3] and in at least 4 animal species, including one gorgonian[15] and 3 mammalian species (see Endogenous DMT).
Another historical milestone is the discovery of DMT in plants frequently used by Amazonian natives as additive to the vine Banisteriopsis caapi to make ayahuasca decoctions. In 1957, American chemists Francis Hochstein and Anita Paradies identify DMT in an "aqueous extract" of leaves of a plant they name Prestonia amazonicum (sic) and describe as "commonly mixed" with B. caapi.[16] The lack of a proper botanical identification of Prestonia amazonica in this study led American ethnobotanist Richard Evans Schultes (1915–2001) and other scientists to raise serious doubts about the claimed plant identity.[17][18] A better evidence is produced in 1965 by French pharmacologist Jacques Poisson who isolates DMT as sole alkaloid from leaves, provided and used by Aguaruna Indians, identified as pertaining to the vine Diplopterys cabrerana (then known as Banisteriopsis rusbyana).[18] Published in 1970, the first identification of DMT in the other commonly used additive plant Psychotria viridis[10] was made by a team of American researchers led by pharmacologist Ara der Marderosian.[19] Not only did they detect DMT in leaves of P. viridis obtained from Cashinahua Indians, but they also were the first to identify it in a sample of an ayahuasca decoction, prepared by the same Indians.[10]
Biosynthesis
 Biosynthetic pathway for N,N-dimethyltryptamine
Biosynthetic pathway for N,N-dimethyltryptamine
Dimethyltryptamine is an indole-alkaloid derived from the shikimate pathway. Its biosynthesis is relatively simple and summarized in the picture to the left. In plants, the parent amino acid L-tryptophan is produced endogenously where in animals L-tryptophan is an essential amino acid coming from diet. No matter the source of L-tryptophan, the biosynthesis begins with its decarboxylation by an aromatic amino acid decarboxylase (AADC) enzyme (step 1). The resulting decarboxylated tryptophan analog is tryptamine. Tryptamine then undergoes a transmethylation (step 2): the enzyme indolethylamine-N-methyltransferase (INMT) catalyzes the transfer of a methyl group from cofactor S-adenosyl-methionine (SAM), via nucleophilic attack, to tryptamine. This reaction transforms SAM into S-adenosylhomocysteine (SAH), and gives the intermediate product N-methyltryptamine (NMT).[20][21] NMT is in turn transmethylated by the same process (step 3) to form the end product N,N-dimethyltryptamine. Tryptamine transmethylation is regulated by two products of the reaction: SAH,[4][22][23] and DMT[4][23] were shown ex vivo to be among the most potent inhibitors of rabbit INMT activity.
This transmethylation mechanism has been repeatedly and consistently proven by radiolabeling of SAM methyl group with carbon-14 (14C-CH3)SAM).[4][20][23][24][25]
Evidence in mammals
Published in Science in 1961, Julius Axelrod found an N-methyltransferase enzyme capable of mediating biotransformation of tryptamine into DMT in a rabbit's lung.[20]This finding initiated a still ongoing scientific interest in endogenous DMT production in humans and other mammals.[21][26]From then on, two major complementary lines of evidence have been investigated: localization and further characterization of the N-methyltransferase enzyme, and analytical studies looking for endogenously produced DMT in body fluids and tissues.[21]
INMT
Before techniques of molecular biology were used to localize indolethylamine N-methyltransferase (INMT),[23][25] characterization and localization went on a par: samples of the biological material where INMT is hypothesized to be active are subject to enzyme assay. Those enzyme assays are performed either with a radiolabeled methyl donor like (14C-CH3)SAM to which known amounts of unlabeled substrates like tryptamine are added,[21] or with addition of a radiolabeled substrate like (14C)NMT to demonstrate in vivo formation.[4][24] As qualitative determination of the radioactively tagged product of the enzymatic reaction is sufficient to characterize INMT existence and activity (or lack of), analytical methods used in INMT assays don't require to be as sensitive as those needed to directly detect and quantify the minute amounts of endogenously formed DMT (see DMT subsection below). The essentially qualitative method thin layer chromatography (TLC) was thus used in a vast majority of studies.[21] Also, robust evidence that INMT can catalyze transmethylation of tryptamine into NMT and DMT could be provided with reverse isotope dilution analysis coupled to mass spectrometry for rabbit[27][28] and human[29] lung during the early 1970s.
Selectivity rather than sensitivity proved to be an Achilles’ heel for some TLC methods with the discovery in 1974-1975 that incubating rat blood cells or brain tissue with (14C-CH3)SAM and NMT as substrate mostly yields tetrahydro-β-carboline derivatives,[4][21][30] and negligible amounts of DMT in brain tissue.[21] It is indeed simultaneously realized that the TLC methods used thus far in almost all published studies on INMT and DMT biosynthesis are incapable to resolve DMT from those tetrahydro-β-carbolines.[21] These findings are a blow for all previous claims of evidence of INMT activity and DMT biosynthesis in avian[31] and mammalian brain,[32][33] including in vivo,[34][35] as they all relied upon use of the problematic TLC methods[21]: their validity is doubted in replication studies that make use of improved TLC methods, and fail to evidence DMT-producing INMT activity in rat and human brain tissues.[36][37] Published in 1978, the last study attempting to evidence in vivo INMT activity and DMT production in brain (rat) with TLC methods finds biotransformation of radiolabeled tryptamine into DMT to be real but "insignificant".[38] Capability of the method used in this latter study to resolve DMT from tetrahydro-β-carbolines is questioned later.[4]
To localize INMT, a qualitative leap is accomplished with use of modern techniques of molecular biology, and of immunohistochemistry. In humans, a gene encoding INMT is determined to be located on chromosome 7.[25] Northern blot analyses reveal INMT messenger RNA (mRNA) to be highly expressed in rabbit lung,[23] and in human thyroid, adrenal gland, and lung.[25][39] Intermediate levels of expression are found in human heart, skeletal muscle, trachea, stomach, small intestine, pancreas, testis, prostate, placenta, lymph node, and spinal cord.[25][39] Low to very low levels of expression are noted in rabbit brain,[25] and human thymus, liver, spleen, kidney, colon, ovary, and bone marrow.[25][39] INMT mRNA expression is absent in human peripheral blood leukocytes, whole brain, and in tissue from 7 specific brain regions (thalamus, subthalamic nucleus, caudate nucleus, hippocampus, amygdala, substantia nigra, and corpus callosum).[25][39] Immunohistochemistry showed INMT to be present in large amounts in glandular epithelial cells of small and large intestines, and to be absent in neurons.[26]Endogenous DMT
The first claimed detection of mammalian endogenous DMT was published in June 1965: German researchers F. Franzen and H. Gross report to have evidenced and quantified DMT, along with its structural analog bufotenin (5-OH-DMT), in human blood and urine.[40] In an article published four months later, the method used in their study is strongly criticized, and credibility of their results challenged.[41]
In 2001, surveys, made in research articles, point that few of the analytical methods previously used to measure levels of endogenously formed DMT had enough sensitivity and selectivity to produce reliable results.[42][43] Gas chromatography, preferably coupled to mass spectrometry (GC-MS), is considered a minimum requirement.[43] A study published in 2005[26] implements the most sensitive and selective method ever used to measure endogenous DMT[44]: liquid chromatography-tandem mass spectrometry with electrospray ionization (LC-ESI-MS/MS) allows to reach limits of detection (LODs) 12 to 200 fold lower (that is, better) than those attained by the best methods employed in the 1970s. The data summarized in the table below are from studies conforming to the abovementioned requirements (abbreviations used: CSF = cerebrospinal fluid; LOD = limit of detection; n = number of samples; ng/L and ng/kg = nanograms (10−9 g) per litre, and nanograms per kilogram, respectively):
DMT in body fluids and tissues (NB: units have been harmonized) Species Sample Results Human Blood serum < LOD (n = 66)[26] Blood plasma < LOD (n = 71)[26] ♦ < LOD (n = 38); 1,000 & 10,600 ng/L (n = 2)[45] Whole blood < LOD (n = 20); 50-790 ng/L (n = 20)[46] Urine < 100 ng/L (n = 9)[26] ♦ < LOD (n = 60); 160-540 ng/L (n = 5)[43] ♦ Detected in n = 10 by GC-MS[47] Feces < 50 ng/kg (n = 12); 130 ng/kg (n = 1)[26] Kidney 15 ng/kg (n = 1)[26] Lung 14 ng/kg (n = 1)[26] Lumbar CSF 100,370 ng/L (n = 1); 2,330-7,210 ng/L (n = 3); 350 & 850 ng/L (n = 2)[48] Rat Kidney 12 &16 ng/kg (n = 2)[26] Lung 22 & 12 ng/kg (n = 2)[26] Liver 6 & 10 ng/kg (n = 2)[26] Brain 10 &15 ng/kg (n = 2)[26] ♦ Measured in synaptic vesicular fraction[49] Rabbit Liver < 10 ng/kg (n = 1)[26] Physical and chemical properties
DMT is commonly handled and stored as a fumarate as other DMT acid salts are generally very hygroscopic and will not readily crystallize. Its freebase form, although less stable than DMT fumarate, is favored by recreational users choosing to vaporize the chemical because it has a lower boiling point. In contrast to DMT's base, its salts are water-soluble. DMT in solution degrades relatively quickly and should be stored protected from air, light, and heat in a freezer.
Pharmacology
Pharmacokinetics
DMT peak levels concentrations (Cmax) measured in whole blood after intramuscular (IM) injection (0.7 mg/kg, n = 11)[50] and in plasma following intravenous (IV) administration (0.4 mg/kg, n = 10)[51] of fully psychedelic doses are in the range of ≈14 to 154 μg/L and 32 to 204 μg/L, respectively. The corresponding molar concentrations of DMT are therefore in the range of 0.074–0.818 µM in whole blood and 0.170–1.08 µM in plasma. However, several studies have described active transport and accumulation of DMT into rat and dog brain following peripheral administration.[52][53][54][55][56] Similar active transport, and accumulation processes likely occur in human brain and may concentrate DMT in brain by several-fold or more (relatively to blood), resulting in local concentrations in the micromolar or higher range. Such concentrations would be commensurate with serotonin brain tissue concentrations which have been consistently determined to be in the 1.5-4 μM range.[57][58]
Closely coextending with peak psychedelic effects, mean time to reach peak concentrations (Tmax) was determined to be 10–15 minutes in whole blood after IM injection,[50] and 2 minutes in plasma after IV administration.[51] When taken orally mixed in an ayahuasca decoction, and in freeze-dried ayahuasca gel caps, DMT Tmax is considerably delayed: 107.59 ± 32.5 minutes,[59] and 90–120 minutes,[60] respectively.Pharmacodynamics
DMT binds non-selectively with affinities < 0.6 μM to the following serotonin receptors: 5-HT1A,[61][62][63] 5-HT1B,[61][64] 5-HT1D,[61][63][64] 5-HT2A,[61][63][64][65] 5-HT2B,[61][64] 5-HT2C,[61][64][65] 5-HT6,[61][64] and 5-HT7.[61][64] An agonist action has been determined at 5-HT1A,[62] 5-HT2A and 5-HT2C.[61][64][65] Its efficacies at other serotonin receptors remain to be determined. Of special interest will be the determination of its efficacy at human 5-HT2B receptor as two in vitro assays evidenced DMT high affinity for this receptor: 0.108 μM[64] and 0.184 μM.[61] This may be of importance because chronic or frequent uses of serotonergic drugs showing preferential high affinity and clear agonism at 5-HT2B receptor have been causally linked to valvular heart disease.[66][67]
It has also been shown to possess affinity for the dopamine D1, α1-adrenergic, α2-adrenergic, imidazoline-1, sigma-1 (σ1), and trace amine-associated receptors.[63][64][68] Agonism was demonstrated at 1 μM at the rat trace amine-associated receptor 1 (TAAR1)[69] and converging lines of evidence established activation of the σ1 receptor at concentrations of 50-100 μM.[70] Its efficacies at the other receptor binding sites are unclear. It has also been shown in vitro to be a substrate for the cell-surface serotonin transporter (SERT) and the intracellular vesicular monoamine transporter 2 (VMAT2), inhibiting SERT-mediated serotonin uptake in human platelets at an average concentration of 4.00 ± 0.70 μM and VMAT2-mediated serotonin uptake in vesicles (of army worm Sf9 cells) expressing rat VMAT-2 at an average concentration of 93 ± 6.8 μM.[71]
Like with other so-called "classical hallucinogens",[72] a large part of DMT psychedelic effects can be attributed to a specific activation of the 5-HT2A receptor.[51][61][73][74][75][76][77] DMT concentrations eliciting 50% of its maximal effect (half maximal effective concentration = EC50 or Kact) at the human 5-HT2A receptor in vitro are in the 0.118-0.983 μM range.[61][64][65][78] This range of values coincides well with the range of concentrations measured in blood and plasma after administration of a fully psychedelic dose (see Pharmacokinetics).
As DMT has been shown to have slightly better efficacy (EC50) at human serotonin 2C receptor than at 2A receptor,[64][65] 5-HT2C highly likely also is implicated in DMT overall effects.[74][79] Other receptors, such as 5-HT1A[63][74][76] σ1,[70][80] and TAAR1[69][81][82] may also play a role.
In 2009 it was hypothesized that DMT may be an endogenous ligand for the σ1 receptor.[70][80] The concentration of DMT needed for σ1 activation in vitro (50-100 μM) is similar to the behaviorally active concentration measured in mouse brain of approximately 106 μM [83] This is minimally 4 orders of magnitude (104) higher than the average concentrations measured in rat brain tissue or human plasma under basal conditions (see Endogenous DMT), so σ1 receptors are likely to be activated only under conditions of high local DMT concentrations. If DMT is stored in synaptic vesicles,[71] such concentrations might occur during vesicular release. To illustrate, while the average concentration of serotonin in brain tissue is in the 1.5-4 μM range,[57][58] the concentration of serotonin in synaptic vesicles was measured at 270 mM.[84] Following vesicular release, the resulting concentration of serotonin in the synaptic cleft, to which serotonin receptors are exposed, is estimated to be about 300 μM. Thus, while in vitro receptor binding affinities, efficacies, and average concentrations in tissue or plasma are useful, they are not likely to predict DMT concentrations in the vesicles or at synaptic or intracellular receptors. Under these conditions, notions of receptor selectivity are moot, and it seems probable that most of the receptors identified as targets for DMT (see above) participate in producing its psychedelic effects.
Psychedelic properties
DMT occurs naturally in many species of plants often in conjunction with its close chemical relatives 5-MeO-DMT and bufotenin (5-OH-DMT).[85] DMT-containing plants are commonly used in South American Shamanic practices. It is usually one of the main active constituents of the drink ayahuasca,[5] however ayahuasca is sometimes brewed with plants which don't produce DMT. It occurs as the primary psychoactive alkaloid in several plants including Mimosa tenuiflora, Diplopterys cabrerana, and Psychotria viridis. DMT is found as a minor alkaloid in snuff made from Virola bark resin in which 5-MeO-DMT is the main active alkaloid.[85] DMT is also found as a minor alkaloid in bark, pods, and beans of Anadenanthera peregrina and Anadenanthera colubrina used to make Yopo and Vilca snuff in which bufotenin is the main active alkaloid.[85][86] Psilocin, an active chemical in many psychedelic mushrooms, is structurally similar to DMT.
The psychotropic effects of DMT were first studied scientifically by the Hungarian chemist and psychologist Dr. Stephen Szára who performed research with volunteers in the mid-1950s. Szára, who later worked for the US National Institutes of Health, had turned his attention to DMT after his order for LSD from the Swiss company Sandoz Laboratories was rejected on the grounds that the powerful psychotropic could be dangerous in the hands of a communist country.[11]
 DMT during various stages of purification
DMT during various stages of purification
DMT can produce powerful entheogenic experiences including intense visuals, euphoria and hallucinations (perceived extensions of reality). DMT is generally not active orally unless it is combined with a monoamine oxidase inhibitor (MAOI) such as a reversible inhibitor of monoamine oxidase A (RIMA), for example, harmaline. Without an MAOI, the body quickly metabolizes orally administered DMT, and it therefore has no hallucinogenic effect unless the dose exceeds monoamine oxidase's metabolic capacity. Other means of ingestion such as smoking or injecting the drug can produce powerful hallucinations and entheogenic activity for a short time (usually less than half an hour), as the DMT reaches the brain before it can be metabolized by the body's natural monoamine oxidase. Taking a MAOI prior to smoking or injecting DMT prolongs and potentiates the effects.[6]
Inhalation
A standard dose for smoked DMT is between 15–60 mg. This is generally smoked in a few successive breaths. The effects last for a short period of time, usually 5 to 15 minutes, dependent on the dose. The onset after inhalation is very fast (less than 45 seconds) and peak effects are reached within a minute. In the 1960s, some reportedly referred to DMT as "the businessman's trip"[87] because of the relatively short duration of vaporized, insufflated, or injected DMT. DMT is commonly vaporized in glass pipes such as those used with crack cocaine and methamphetamine. The vapor is sometimes described as harsh, and some users even compare its flavor to that of burning plastic. Combining DMT with plant matter or depositing it upon a substrate of ash also facilitates use of an ordinary smoking pipe or a vaporizer.
Insufflation
Insufflating DMT (commonly as a freebase or fumarate) requires a higher dose than inhalation. The duration is markedly increased, and some users report diminished euphoria but an intensified otherworldly experience.[citation needed]
Injection
Injected DMT produces an experience that is similar to inhalation in duration, intensity, and characteristics.
In a study conducted from 1990 through 1995, University of New Mexico psychiatrist Rick Strassman found that some volunteers injected with high doses of DMT had experiences with a perceived alien entity. Usually, the reported entities were experienced as the inhabitants of a perceived independent reality the subjects reported visiting while under the influence of DMT.[11] In a September, 2009, interview with Examiner.com, Strassman described the effects on participants in the study: "Subjectively, the most interesting results were that high doses of DMT seemed to allow the consciousness of our volunteers to enter into non-corporeal, free-standing, independent realms of existence inhabited by beings of light who oftentimes were expecting the volunteers, and with whom the volunteers interacted. While 'typical' near-death and mystical states occurred, they were relatively rare."
Oral ingestion
DMT is broken down by the digestive enzyme monoamine oxidase and is practically inactive if taken orally, unless combined with an MAOI. The traditional South American ayahuasca, or yage, is a tea mixture containing DMT and a MAOI.[5] There are a variety of recipes to this brew, but most commonly it is simply the leaves of Psychotria viridis (the source of DMT) and the vine Banisteriopsis caapi (the source of MAOI). Other DMT containing plants, including Diplopterys cabrerana, are sometimes used in ayahuasca in different areas of South America. Two common sources in the western US are Reed canary grass (Phalaris arundinacea) and Harding grass (Phalaris aquatica). These invasive grasses contain low levels of DMT and other alkaloids. In addition, Jurema (Mimosa tenuiflora) shows evidence of DMT content: the pink layer in the bark of this vine contains a high concentration of N,N-DMT.
Taken orally with an appropriate MAOI, DMT produces a long lasting (over 3 hour), slow, deep metaphysical experience similar to that of psilocybin mushrooms, but more intense.[5] MAOIs should be used with extreme caution as they can have lethal complications with some prescription drugs such as SSRI antidepressants, some over-the-counter drugs,[88] and many common foods.[89]
Induced DMT experiences can include profound time-dilation, visual and auditory illusions, and other experiences that, by most firsthand accounts, defy verbal or visual description. Some users report intense erotic imagery and sensations and utilize the drug in a ritual sexual context.[5][90][91]
Distinguish from 5-MeO-DMT
5-MeO-DMT, a psychedelic drug structurally similar to N,N-DMT, is sometimes referred to as DMT through ignorance or by abbreviation. As a white, crystalline solid, it is also similar in appearance to DMT. However, it is considerably more potent (5-MeO-DMT typical smoked dose: 5–20 mg), and care should be taken to clearly differentiate between the two drugs to avoid accidental overdose.[92]
Detection in body fluids
DMT may be quantitated in blood, plasma or urine using chromatographic techniques as a diagnostic tool in clinical poisoning situations or to aid in the medicolegal investigation of suspicious deaths. Blood or plasma DMT levels in recreational users of the drug are generally in the 10-30 μg/L range during the first several hours post-ingestion. Less than 0.1% of an oral dose is eliminated unchanged in the 24 hour urine of humans.[93][94]
Side effects
Similar to other psychedelic drugs, there are relatively few physical side effects associated with DMT acute exposure. When inhaled, its vapor has been described as "very harsh."[95] According to a "Dose-response study of N,N-dimethyltryptamine in humans" by Rick Strassman, "Dimethyltryptamine dose slightly elevated blood pressure, heart rate, pupil diameter, and rectal temperature, in addition to elevating blood concentrations of beta-endorphin, corticotropin, cortisol, and prolactin. Growth hormone blood levels rose equally in response to all doses of DMT, and melatonin levels were unaffected."[51]
Psychologically, the DMT experience can be overly-intense, potentially causing overwhelming fear and difficulty integrating experiences if one is not mentally prepared. Furthermore, due to the intense nature of the experience, DMT is generally considered to have no addiction potential.[citation needed] The fear that may come as a response to the uncontrollable nature of the drug can be dealt with by mental preparation and understanding how the drug will affect the mind and body. Knowing what is coming before dosing can make the experience less fearsome.[6] Because the effects are so short-lived, it has been referred to as the "businessman's trip", implying that the trip lasts no longer than a lunch break.[96]
Conjecture
Several speculative and yet untested hypotheses suggest that endogenous DMT is produced in the human brain and is involved in certain psychological and neurological states. DMT is naturally occurring in small amounts in rat brain, human cerebrospinal fluid, and other tissues of humans and other mammals. It may play a role in mediating the visual effects of natural dreaming, and also near-death experiences, religious visions and other mystical states.[97] A biochemical mechanism for this was proposed by the medical researcher J. C. Callaway, who suggested in 1988 that DMT might be connected with visual dream phenomena: brain DMT levels would be periodically elevated to induce visual dreaming and possibly other natural states of mind.[98] A new hypothesis proposed is that in addition to being involved in altered states of consciousness, endogenous DMT may be involved in the creation of normal waking states of consciousness. It is proposed that DMT and other endogenous hallucinogens mediate their neurological abilities by acting as neurotransmitters at a sub class of the trace amine receptors; a group of receptors found in the CNS where DMT and other hallucinogens have been shown to have activity. Wallach further proposes that in this way waking consciousness can be thought of as a controlled psychedelic experience. It is when the control of these systems becomes loosened and their behavior no longer correlates with the external world that the altered states arise.[81]
Dr. Rick Strassman, while conducting DMT research in the 1990s at the University of New Mexico, advanced the controversial hypothesis that a massive release of DMT from the pineal gland prior to death or near death was the cause of the near death experience (NDE) phenomenon. Several of his test subjects reported NDE-like audio or visual hallucinations. His explanation for this was the possible lack of panic involved in the clinical setting and possible dosage differences between those administered and those encountered in actual NDE cases. Several subjects also reported contact with 'other beings', alien like, insectoid or reptilian in nature, in highly advanced technological environments[11] where the subjects were 'carried,' 'probed,' 'tested,' 'manipulated,' 'dismembered,' 'taught,' 'loved,' and even 'raped' by these 'beings' (one could note the strong similarities of these bodily tests/invasions in other psychedelic experiences throughout time, outlined in Graham Hancock's "Supernatural"[99]). Basing his reasoning on the unreferenced and unsupported statement that all the enzymatic material needed to produce DMT is found in the pineal gland (see evidence in mammals), and moreover in substantially greater concentrations than in any other part of the body, Strassman ([11] p. 69) has speculated that DMT is made in the pineal gland. Currently there is no published reliable scientific evidence supporting this hypothesis and as such, it is merely a hypothesis.
In the 1950s, the endogenous production of psychoactive agents was considered to be a potential explanation for the hallucinatory symptoms of some psychiatric diseases as the transmethylation hypothesis[100] (see also adrenochrome), though this hypothesis does not account for the natural presence of endogenous DMT in otherwise normal humans, rats and other laboratory animals.
Writers on DMT include Terence McKenna, Jeremy Narby and Graham Hancock. In his writings and speeches, Terence McKenna recounts encounters with entities he sometimes describes as "Self-Transforming Machine Elves" among other phrases. McKenna believed DMT to be a tool that could be used to enhance communication and allow for communication with other-worldly entities. Other users report visitation from external intelligences attempting to impart information.
Legal status
International Law
DMT is classified as a Schedule I drug under the UN 1971 Convention on Psychotropic Substances, meaning that use of DMT is supposed to be restricted to scientific research and medical use and international trade in DMT is supposed to be closely monitored. Natural materials containing DMT, including ayahuasca, are explicitly not regulated under the 1971 Psychotropic Convention.[101]
Australia
The Australian Federal Government is considering changes to the Australian Criminal Code that would classify any plants containing any amount of DMT as "controlled plants".[102]
Canada
DMT is classified in Canada as a Schedule III drug.
France
DMT, along with most of its plant sources, is classified in France as a stupéfiant (narcotic).
New Zealand
DMT is classified in New Zealand as a Class A drug.[103]
United Kingdom
DMT is classified in the United Kingdom as a Class A drug.
United States
DMT is classified in the United States as a Schedule I drug under the Controlled Substances Act of 1970.
In December 2004, the Supreme Court lifted a stay thereby allowing the Brazil-based União do Vegetal (UDV) church to use a decoction containing DMT in their Christmas services that year. This decoction is a tea made from boiled leaves and vines, known as hoasca within the UDV, and ayahuasca in different cultures. In Gonzales v. O Centro Espirita Beneficente Uniao do Vegetal, the Supreme Court heard arguments on November 1, 2005 and unanimously ruled in February 2006 that the U.S. federal government must allow the UDV to import and consume the tea for religious ceremonies under the 1993 Religious Freedom Restoration Act.
In September, 2008, the three Santo Daime churches filed suit in federal court to gain legal status to import DMT-containing ayahuasca tea. The case, Church of the Holy Light of the Queen v. Mukasey,[104] presided over by Judge Owen M. Panner, was ruled in favor of the Santo Daime church. As of March 21, 2009 a federal judge says members of the church in Ashland can import, distribute and brew ayahuasca. U.S. District Judge Owen Panner issued a permanent injunction barring the government from prohibiting or penalizing the sacramental use of "Daime tea." Panner's order said activities of The Church of the Holy Light of the Queen are legal and protected under freedom of religion. His order prohibits the federal government from interfering with and prosecuting church members who follow a list of regulations set out in his order.[105]
Culture
In South America there are a number of indigenous traditions and more recent religious movements based on the use of ayahuasca, usually in an animistic context that may be mixed with Christian imagery. There are four main branches using DMT-MAOI based sacraments in South America:
- The Amazon Basin's indigenous population. There are many indigenous cultures in South America, mostly in the Upper Amazon Basin whose traditional religious practices include the use of ayahuasca. These are the oldest cultures in the whole of South America that continue to use ayahuasca or analogue brews, such as the ones made from Jurema in the Pernambuco, near Recife or Iquitos in Peru.
- Santo Daime ("Saint Give Unto Me") and Barquinha ("Little Boat"). A syncretic religion from Brazil. The former was founded by Raimundo Irineu Serra in the early 1930s, as an esoteric Christian religion with shamanic tendencies. The Barquinha was derived from this one. The Santo Daime also includes children in their Entheogenic rituals, studies done by the Brazilian government concluded that there were no physical or mental damage caused by this practice so it is allowed.
- União do Vegetal ("Union of the Plants" or UDV). Another Christian ayahuasca religion from Brazil, a single unified organization with a democratic structure.
- Neo-shamans. There are some self-styled shamanic facilitators in Brazil and other South American countries that use ayahuasca or analogous brews in their rituals and séances.
See also
References
- ^ Häfelinger, G.; Nimtz, M.; Horstmann, V.; Benz, T. (1999). "Untersuchungen zur Trifluoracetylierung der Methylderivate von Tryptamin und Serotonin mit verschiedenen Derivatisierungsreagentien: Synthesen, Spektroskopie sowie analytische Trennungen mittels Kapillar-GC [Trifluoracetylation of methylated derivatives of tryptamine and serotonin by different reagents: synthesis, spectroscopic characterizations, and separations by capillary-gas-chromatography]". Zeitschrift für Naturforschung B 54 (3): 397–414.
- ^ Corothie, E; Nakano, T (1969). "Constituents of the bark of Virola sebifera". Planta Medica 17 (2): 184–188. doi:10.1055/s-0028-1099844. PMID 5792479.
- ^ a b Ott, Jonathan (1994). Ayahuasca Analogues: Pangæan Entheogens. Kennewick, WA: Natural Products. pp. 81–83. ISBN 978-0961423452.
- ^ a b c d e f g Barker S.A., Monti J.A., Christian S.T. (1981). "N, N-dimethyltryptamine: an endogenous hallucinogen". International Review of Neurobiology. International Review of Neurobiology 22: 83–110. doi:10.1016/S0074-7742(08)60291-3. ISBN 9780123668226. PMID 6792104.
- ^ a b c d e Salak, Kira. "Hell and back". National Geographic Adventure. http://www.kirasalak.com/Peru.html.
- ^ a b c http://www.erowid.org/chemicals/dmt/dmt.shtml
- ^ McKenna D.J., Towers G.H.N., Abbott F. (April 1984). "Monoamine oxidase inhibitors in South American hallucinogenic plants: tryptamine and β-carboline constituents of ayahuasca" (PDF). Journal of Ethnopharmacology 10 (2): 195–223. doi:10.1016/0378-8741(84)90003-5. PMID 6587171. http://crfdl.org:1111/xmlui/bitstream/handle/123456789/280/DennisJEthn84_2.pdf.
- ^ Manske R.H.F. (1931). "A synthesis of the methyltryptamines and some derivatives". Canadian Journal of Research 5 (5): 592–600. doi:10.1139/cjr31-097. http://rparticle.web-p.cisti.nrc.ca/rparticle/AbstractTemplateServlet?calyLang=eng&journal=cjr&volume=5&year=&issue=5&msno=cjr31-097.
- ^ a b Bigwood J., Ott J. (November 1977). "DMT: the fifteen minute trip". Head 2 (4): 56–61. Archived from the original on 2006-01-27. http://web.archive.org/web/20060127003553/http://jeremybigwood.net/JBsPUBS/DMT/. Retrieved 2010-11-28.
- ^ a b c Ott, Jonathan (1996). Pharmacotheon: Entheogenic Drugs, Their Plant Sources and History (2nd, densified ed.). Kennewick, WA: Natural Products. ISBN 978-0961423490.
- ^ a b c d e Strassman, Rick J. (2001). DMT: The Spirit Molecule. A Doctor's Revolutionary Research into the Biology of Near-Death and Mystical Experiences. Rochester, Vt: Park Street. ISBN 978-0892819270. ("Chapter summaries". Archived from the original on 2007-01-09. http://web.archive.org/web/20070109211058/http://www.rickstrassman.com/dmt/chaptersummaries.html. Retrieved 2007-01-13.)
- ^ a b c Ott, Jonathan (1998). "Pharmahuasca, anahuasca and vinho da jurema: human pharmacology of oral DMT plus harmine". In Müller-Ebeling, C.. Special: Psychoactivity. Yearbook for Ethnomedicine and the Study of Consciousness. 6/7 (1997/1998). Berlin: VWB. ISBN 3-86135-033-5. https://www.erowid.org/references/texts/show/7105docid6446.
- ^ Pachter I.J., Zacharias D.E., Ribeiro O. (September 1959). "Indole alkaloids of Acer saccharinum (the silver maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis". Journal of Organic Chemistry 24 (9): 1285–87. doi:10.1021/jo01091a032.
- ^ Fish M.S., Johnson N.M., Horning E.C. (November 1955). "Piptadenia alkaloids. Indole bases of P. peregrina (L.) Benth. and related species". Journal of the American Chemical Society 72 (22): 5892–95. doi:10.1021/ja01627a034.
- ^ Cimino G., De Stefano S. (1978). "Chemistry of Mediterranean gorgonians: simple indole derivatives from Paramuricea chamaeleon". Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 61 (2): 361–2. doi:10.1016/0306-4492(78)90070-9.
- ^ Hochstein F.A., Paradies A.M. (1957). "Alkaloids of Banisteria caapi and Prestonia amazonicum". Journal of the American Chemical Society 79 (21): 5735–36. doi:10.1021/ja01578a041. http://pubs.acs.org/doi/abs/10.1021/ja01578a041.
- ^ Schultes R.E., Raffauf R.F. (1960). "Prestonia: An Amazon narcotic or not?". Botanical Museum Leaflets, Harvard University 19 (5): 109–122. ISSN 0006-8098. http://www.biodiversitylibrary.org/item/31906#page/126/mode/1up.
- ^ a b Poisson J. (April 1965). "Note sur le "Natem", boisson toxique péruvienne et ses alcaloïdes [Note on "Natem", a toxic Peruvian beverage, and its alkaloids]" (in French). Annales Pharmaceutiques Françaises 23: 241–4. ISSN 0003-4509. PMID 14337385.
- ^ Der Marderosian A.H., Kensinger K.M., Chao J.-M., Goldstein F.J. (1970). "The use and hallucinatory principles of a psychoactive beverage of the Cashinahua tribe (Amazon basin)". Drug Dependence 5: 7–14. ISSN 0070-7368. OCLC 1566975.
- ^ a b c Axelrod J. (August 1961). "Enzymatic formation of psychotomimetic metabolites from normally occurring compounds". Science 134 (3475): 343. doi:10.1126/science.134.3475.343. PMID 13685339.
- ^ a b c d e f g h i Rosengarten H., Friedhoff A.J. (1976). "A review of recent studies of the biosynthesis and excretion of hallucinogens formed by methylation of neurotransmitters or related substances" (PDF). Schizophrenia Bulletin 2 (1): 90–105. doi:10.1093/schbul/2.1.90. PMID 779022. http://schizophreniabulletin.oxfordjournals.org/content/2/1/90.full.pdf.
- ^ Lin R.L., Narasimhachari N., Himwich H.E. (September 1973). "Inhibition of indolethylamine-N-methyltransferase by S-adenosylhomocysteine". Biochemical and Biophysical Research Communications 54 (2): 751–9. doi:10.1016/0006-291X(73)91487-3. PMID 4756800.
- ^ a b c d e Thompson M.A., Weinshilboum R.M. (December 1998). "Rabbit lung indolethylamine N-methyltransferase. cDNA and gene cloning and characterization". Journal of Biological Chemistry 273 (51): 34502–10. doi:10.1074/jbc.273.51.34502. PMID 9852119. http://www.jbc.org/content/273/51/34502.long. Retrieved 2010-11-09.
- ^ a b Mandel L.R., Prasad R., Lopez-Ramos B., Walker R.W. (January 1977). "The biosynthesis of dimethyltryptamine in vivo". Research Communications in Chemical Pathology and Pharmacology 16 (1): 47–58. PMID 14361.
- ^ a b c d e f g h Thompson M.A., Moon E., Kim U.J., Xu J., Siciliano M.J., Weinshilboum R.M. (November 1999). "Human indolethylamine N-methyltransferase: cDNA cloning and expression, gene cloning, and chromosomal localization" (PDF). Genomics 61 (3): 285–97. doi:10.1006/geno.1999.5960. PMID 10552930. http://crfdl.org:1111/xmlui/bitstream/handle/123456789/307/Thompson99humanINMT.pdf?sequence=1.
- ^ a b c d e f g h i j k l m n Kärkkäinen J., Forsström T., Tornaeus J., Wähälä K., Kiuru P., Honkanen A., Stenman U.-H., Turpeinen U., Hesso A. (April 2005). "Potentially hallucinogenic 5-hydroxytryptamine receptor ligands bufotenine and dimethyltryptamine in blood and tissues". Scandinavian Journal of Clinical and Laboratory Investigation 65 (3): 189–199. doi:10.1080/00365510510013604. PMID 16095048.
- ^ Mandel L.R., Rosenzweig S., Kuehl F.A. (March 1971). "Purification and substrate specificity of indoleamine-N-methyl transferase". Biochemical Pharmacology 20 (3): 712–6. doi:10.1016/0006-2952(71)90158-4. PMID 5150167.
- ^ Lin R.-L., Narasimhachari N. (June 1975). "N-methylation of 1-methyltryptamines by indolethylamine N-methyltransferase". Biochemical Pharmacology 24 (11–12): 1239–40. doi:10.1016/0006-2952(75)90071-4. PMID 1056183.
- ^ Mandel L.R., Ahn H.S., VandenHeuvel W.J. (April 1972). "Indoleamine-N-methyl transferase in human lung". Biochemical Pharmacology 21 (8): 1197–200. doi:10.1016/0006-2952(72)90113-X. PMID 5034200.
- ^ Rosengarten H., Meller E., Friedhoff A.J. (1976). "Possible source of error in studies of enzymatic formation of dimethyltryptamine". Journal of Psychiatric Research 13 (1): 23–30. doi:10.1016/0022-3956(76)90006-6. PMID 1067427.
- ^ Morgan M., Mandell A.J. (August 1969). "Indole(ethyl)amine N-methyltransferase in the brain". Science 165 (3892): 492–3. doi:10.1126/science.165.3892.492. PMID 5793241.
- ^ Mandell A.J., Morgan M. (March 1971). "Indole(ethyl)amine N-methyltransferase in human brain". Nature: New Biology 230 (11): 85–7. doi:10.1038/newbio230085a0. PMID 5279043.
- ^ Saavedra J.M., Coyle J.T., Axelrod J. (March 1973). "The distribution and properties of the nonspecific N-methyltransferase in brain". Journal of Neurochemistry 20 (3): 743–52. doi:10.1111/j.1471-4159.1973.tb00035.x. PMID 4703789.
- ^ Saavedra J.M., Axelrod J. (March 1972). "Psychotomimetic N-methylated tryptamines: formation in brain in vivo and in vitro" (PDF). Science 175 (4028): 1365–6. doi:10.1126/science.175.4028.1365. PMID 5059565. http://crfdl.org:1111/xmlui/bitstream/handle/123456789/392/1733285.pdf?sequence=1.
- ^ Wu P.H., Boulton A.A. (July 1973). "Distribution and metabolism of tryptamine in rat brain". Canadian Journal of Biochemistry 51 (7): 1104–12. doi:10.1139/o73-144. PMID 4725358.
- ^ Boarder M.R., Rodnight R. (September 1976). "Tryptamine-N-methyltransferase activity in brain tissue: a re-examination". Brain Research 114 (2): 359–64. doi:10.1016/0006-8993(76)90680-6. PMID 963555.
- ^ Gomes U.R., Neethling A.C., Shanley B.C. (September 1976). "Enzymatic N-methylation of indoleamines by mammalian brain: fact or artefact?". Journal of Neurochemistry 27 (3): 701–5. doi:10.1111/j.1471-4159.1976.tb10397.x. PMID 823298.
- ^ Stramentinoli G., Baldessarini R.J. (October 1978). "Lack of enhancement of dimethyltryptamine formation in rat brain and rabbit lung in vivo by methionine or S-adenosylmethionine". Journal of Neurochemistry 31 (4): 1015–20. doi:10.1111/j.1471-4159.1978.tb00141.x. PMID 279646.
- ^ a b c d General annotation of Human INMT (O95050) entry in UniProtKB/Swiss-Prot
- ^ Franzen F., Gross H. (June 1965). "Tryptamine, N,N-dimethyltryptamine, N,N-dimethyl-5-hydroxytryptamine and 5-methoxytryptamine in human blood and urine". Nature 206 (988): 1052. doi:10.1038/2061052a0. PMID 5839067. "After the elaboration of sufficiently selective and quantitative procedures, which are discussed elsewhere, we were able to study the occurrence of tryptamine, N,N-dimethyltryptamine, N,N-dimethyl-5-hydroxytryptamine and 5-hydroxytryptamine in normal human blood and urine. (...) In 11 of 37 probands N,N-dimethyltryptamine was demonstrated in blood (...). In the urine 42·95 ± 8·6 μg of dimethyltryptamine/24 h were excreted."
- ^ Siegel M. (October 1965). "A sensitive method for the detection of N,N-dimethylserotonin (bufotenin) in urine; failure to demonstrate its presence in the urine of schizophrenic and normal subjects". Journal of Psychiatric Research 3 (3): 205–11. doi:10.1016/0022-3956(65)90030-0. PMID 5860629.
- ^ Barker S.A., Littlefield-Chabaud M.A., David C. (February 2001). "Distribution of the hallucinogens N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine in rat brain following intraperitoneal injection: application of a new solid-phase extraction LC-APcI-MS-MS-isotope dilution method". Journal of Chromatography B 751 (1): 37–47. doi:10.1016/S0378-4347(00)00442-4. PMID 11232854.
- ^ a b c Forsström T., Tuominen J., Karkkäinen J. (2001). "Determination of potentially hallucinogenic N-dimethylated indoleamines in human urine by HPLC/ESI-MS-MS". Scandinavian Journal of Clinical and Laboratory Investigation 61 (7): 547–56. doi:10.1080/003655101753218319. PMID 11763413.
- ^ Shen H.W., Jiang X.L., Yu A.M. (April 2009). "Development of a LC-MS/MS method to analyze 5-methoxy-N,N-dimethyltryptamine and bufotenine, and application to pharmacokinetic study". Bioanalysis 1 (1): 87–95. doi:10.4155/bio.09.7. PMC 2879651. PMID 20523750. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2879651.
- ^ Wyatt R.J., Mandel L.R., Ahn H.S., Walker R.W., Vanden Heuvel W.J. (July 1973). "Gas chromatographic-mass spectrometric isotope dilution determination of N,N-dimethyltryptamine concentrations in normals and psychiatric patients" (PDF). Psychopharmacologia 31 (3): 265–70. doi:10.1007/BF00422516. PMID 4517484. http://www.springerlink.com/content/j686565850024164/fulltext.pdf.
- ^ Angrist B., Gershon S., Sathananthan G., Walker R.W., Lopez-Ramos B., Mandel L.R., Vandenheuvel W.J. (May 1976). %7CFormat PDF "Dimethyltryptamine levels in blood of schizophrenic patients and control subjects". Psychopharmacology 47 (1): 29–32. doi:10.1007/BF00428697. PMID 803203. http://www.springerlink.com/content/kw2nm252m3248864/fulltext.pdf %7CFormat PDF.
- ^ Oon M.C., Rodnight R. (December 1977). "A gas chromatographic procedure for determining N, N-dimethyltryptamine and N-monomethyltryptamine in urine using a nitrogen detector". Biochemical Medicine 18 (3): 410–9. doi:10.1016/0006-2944(77)90077-1. PMID 271509.
- ^ Smythies J.R., Morin R.D., Brown G.B. (June 1979). "Identification of dimethyltryptamine and O-methylbufotenin in human cerebrospinal fluid by combined gas chromatography/mass spectrometry". Biological Psychiatry 14 (3): 549–56. PMID 289421.
- ^ Christian S.T., Harrison R., Quayle E., Pagel J., Monti J. (October 1977). "The in vitro identification of dimethyltryptamine (DMT) in mammalian brain and its characterization as a possible endogenous neuroregulatory agent". Biochemical Medicine 18 (2): 164–83. doi:10.1016/0006-2944(77)90088-6. PMID 20877.
- ^ a b Kaplan J., Mandel L.R., Stillman R., Walker R.W., VandenHeuvel W.J., Gillin J.C., Wyatt R.J. (1974). "Blood and urine levels of N,N-dimethyltryptamine following administration of psychoactive dosages to human subjects" (PDF). Psychopharmacologia 38 (3): 239–45. doi:10.1007/BF00421376. PMID 4607811. http://www.springerlink.com/content/v22655wm10341746/fulltext.pdf.
- ^ a b c d Strassman R.J., Qualls C.R. (February 1994). "Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects". Archives of General Psychiatry 51 (2): 85–97. PMID 8297216.
- ^ Barker S.A., Beaton J.M., Christian S.T., Monti J.A., Morris P.E. (August 1982). "Comparison of the brain levels of N,N-dimethyltryptamine and α, α, β, β-tetradeutero-N-N-dimethyltryptamine following intraperitoneal injection. The in vivo kinetic isotope effect". Biochemical Pharmacology 31 (15): 2513–6. doi:10.1016/0006-2952(82)90062-4. PMID 6812592.
- ^ Sangiah S., Gomez M.V., Domino E.F. (December 1979). "Accumulation of N,N-dimethyltryptamine in rat brain cortical slices". Biological Psychiatry 14 (6): 925–36. PMID 41604.
- ^ Sitaram B.R., Lockett L., Talomsin R., Blackman G.L., McLeod W.R. (May 1987). "In vivo metabolism of 5-methoxy-N,N-dimethyltryptamine and N,N-dimethyltryptamine in the rat". Biochemical Pharmacology 36 (9): 1509–12. doi:10.1016/0006-2952(87)90118-3. PMID 3472526.
- ^ Takahashi T., Takahashi K., Ido T., Yanai K., Iwata R., Ishiwata K., Nozoe S. (December 1985). "[11C]-labeling of indolealkylamine alkaloids and the comparative study of their tissue distributions". International Journal of Applied Radiation and Isotopes 36 (12): 965–9. doi:10.1016/0020-708X(85)90257-1. PMID 3866749.
- ^ Yanai K., Ido T., Ishiwata K., Hatazawa J, Takahashi T., Iwata R., Matsuzawa T. (1986). "In vivo kinetics and displacement study of a carbon-11-labeled hallucinogen, N,N-(11C)dimethyltryptamine" (PDF). European Journal of Nuclear Medicine 12 (3): 141–6. doi:10.1007/BF00276707. PMID 3489620. http://www.springerlink.com/content/j2l4821226141002/fulltext.pdf.
- ^ a b Best J, Nijhout HF, Reed M (2010). "Serotonin synthesis, release and reuptake in terminals: a mathematical model". Theoretical Biology & Medical Modelling 7: 34. doi:10.1186/1742-4682-7-34. PMC 2942809. PMID 20723248. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2942809.
- ^ a b Merrill MA, Clough RW, Jobe PC, Browning RA (September 2005). "Brainstem seizure severity regulates forebrain seizure expression in the audiogenic kindling model" (PDF). Epilepsia 46 (9): 1380–8. doi:10.1111/j.1528-1167.2005.39404.x. PMID 16146432. http://assets0.pubget.com/pdf/16146432.pdf.
- ^ Callaway J.C., McKenna D.J., Grob C.S., Brito G.S., Raymon L.P., Poland R.E., Andrade E.N. et al. (June 1999). "Pharmacokinetics of Hoasca alkaloids in healthy humans" (PDF). Journal of Ethnopharmacology 65 (3): 243–56. doi:10.1016/S0378-8741(98)00168-8. PMID 10404423. http://wiki.dmt-nexus.com/w/images/2/26/Pharmacokinetics_of_hoasca_in_healthy_humans.pdf.
- ^ Riba J., Valle M., Urbano G., Yritia M., Morte A., Barbanoj M.J. (July 2003). "Human pharmacology of ayahuasca: subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics" (PDF). Journal of Pharmacology and Experimental Therapeutics 306 (1): 73–83. doi:10.1124/jpet.103.049882. PMID 12660312. http://jpet.aspetjournals.org/content/306/1/73.full.pdf.
- ^ a b c d e f g h i j k l Keiser M.J., Setola V., Irwin J.J., Laggner C., Abbas A.I., Hufeisen S.J., Jensen N.H. et al. (November 2009). "Predicting new molecular targets for known drugs". Nature 462 (7270): 175–81. doi:10.1038/nature08506. PMC 2784146. PMID 19881490. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2784146.
- ^ a b Deliganis A.V., Pierce P.A., Peroutka S.J. (June 1991). "Differential interactions of dimethyltryptamine (DMT) with 5-HT1A and 5-HT2 receptors". Biochemical Pharmacology 41 (11): 1739–44. doi:10.1016/0006-2952(91)90178-8. PMID 1828347.
- ^ a b c d e Pierce P.A., Peroutka S.J. (1989). "Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex" (PDF). Psychopharmacology 97 (1): 118–22. doi:10.1007/BF00443425. PMID 2540505. http://www.springerlink.com/content/p071q46411657071/fulltext.pdf.
- ^ a b c d e f g h i j k l Ray T.S. (2010). Manzoni, Olivier Jacques. ed. "Psychedelics and the Human Receptorome". PLoS ONE 5 (2): e9019. doi:10.1371/journal.pone.0009019. PMC 2814854. PMID 20126400. http://dx.plos.org/10.1371/journal.pone.0009019.
- ^ a b c d e Smith R.L., Canton H., Barrett R.J., Sanders-Bush E. (November 1998). "Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors" (PDF). Pharmacology, Biochemistry, and Behavior 61 (3): 323–30. doi:10.1016/S0091-3057(98)00110-5. PMID 9768567. http://crfdl.org:1111/xmlui/bitstream/handle/123456789/17/Agonist%20Properties%20of%20N,N-Dimethyltryptaminenext%20term%20at%20Ser.pdf.
- ^ Rothman R.B., Baumann M.H. (May 2009). "Serotonergic Drugs and Valvular Heart Disease" (PDF). Expert Opinion on Drug Safety 8 (3): 317–29. doi:10.1517/14740330902931524. PMC 2695569. PMID 19505264. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2695569.
- ^ Roth B.L. (January 2007). "Drugs and valvular heart disease". New England Journal of Medicine 356 (1): 6–9. doi:10.1056/NEJMp068265. PMID 17202450.
- ^ Burchett S.A., Hicks T.P. (August 2006). "The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain". Progress in Neurobiology 79 (5–6): 223–46. doi:10.1016/j.pneurobio.2006.07.003. PMID 16962229.
- ^ a b Bunzow J.R., Sonders M.S., Arttamangkul S., Harrison L.M., Zhang G., Quigley D.I., Darland T.et al. (2001). "Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor" (PDF). Molecular Pharmacology 60 (6): 1181–8. PMID 11723224. http://molpharm.aspetjournals.org/content/60/6/1181.full.pdf.
- ^ a b c Fontanilla D., Johannessen M., Hajipour A.R., Cozzi N.V., Jackson M.B., Ruoho A.E. (February 2009). "The Hallucinogen N,N-Dimethyltryptamine (DMT) Is an Endogenous Sigma-1 Receptor Regulator". Science 323 (5916): 934–7. doi:10.1126/science.1166127. PMC 2947205. PMID 19213917. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=19213917.
- ^ a b Cozzi N.V., Gopalakrishnan A., Anderson L.L., Feih J.T., Shulgin A.T., Daley P.F., Ruoho A.E. (December 2009). "Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter" (PDF). Journal of Neural Transmission 116 (12): 1591–9. doi:10.1007/s00702-009-0308-8. PMID 19756361. http://www.neurophys.wisc.edu/~cozzi/Hallucinogenic%20tryptamines%20as%20SERT%20and%20VMAT2%20substrates.%20%20Cozzi.%20%20J.%20Neural%20Transm.,%20116,%201591-1599%20(2009).pdf.
- ^ Glennon, R.A. (1994). "Classical hallucinogens: an introductory overview". In Lin, G.C.; Glennon, R.A.. Hallucinogens: An Update. NIDA Research Monograph Series. 146. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Drug Abuse. p. 4. http://crfdl.org:1111/xmlui/bitstream/handle/123456789/288/hallucinogens%20an%20update.pdf.
- ^ Fantegrossi W.E., Murnane K.S., Reissig C.J. (January 2008). "The behavioral pharmacology of hallucinogens" (PDF). Biochemical Pharmacology 75 (1): 17–33. doi:10.1016/j.bcp.2007.07.018. PMC 2247373. PMID 17977517. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2247373.
- ^ a b c Nichols D.E. (February 2004). "Hallucinogens". Pharmacology & Therapeutics 101 (2): 131–81. doi:10.1016/j.pharmthera.2003.11.002. PMID 14761703.
- ^ Vollenweider F.X., Vollenweider-Scherpenhuyzen M.F., Bäbler A., Vogel H., Hell D. (December 1998). "Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action". Neuroreport 9 (17): 3897–902. doi:10.1097/00001756-199812010-00024. PMID 9875725.
- ^ a b Strassman R.J. (1996). "Human psychopharmacology of N,N-dimethyltryptamine" (PDF). Behavioural Brain Research 73 (1–2): 121–4. doi:10.1016/0166-4328(96)00081-2. PMID 8788488. http://crfdl.org:1111/xmlui/bitstream/handle/123456789/373/Beh_Brain_Res_96.pdf.
- ^ Glennon R.A., Titeler M., McKenney J.D. (December 1984). "Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents". Life Sciences 35 (25): 2505–11. doi:10.1016/0024-3205(84)90436-3. PMID 6513725.
- ^ Roth B.L., Choudhary M.S., Khan N., Uluer A.Z. (February 1997). "High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model" (PDF). Journal of Pharmacology and Experimental Therapeutics 280 (2): 576–83. PMID 9023266. http://jpet.aspetjournals.org/content/280/2/576.full.pdf.
- ^ Canal C.E., Olaghere da Silva U.B., Gresch P.J., Watt E.E., Sanders-Bush E., Airey D.C. (April 2010). "The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen" (PDF). Psychopharmacology 209 (2): 163–74. doi:10.1007/s00213-010-1784-0. PMC 2868321. PMID 20165943. http://www.springerlink.com/content/614528n2772tr715/fulltext.pdf.
- ^ a b Su T.P., Hayashi T., Vaupel D.B. (2009). "When the Endogenous Hallucinogenic Trace Amine N,N-Dimethyltryptamine Meets the Sigma-1 Receptor" (PDF). Science Signaling 2 (61): pe12. doi:10.1126/scisignal.261pe12. PMC 3155724. PMID 19278957. http://crfdl.org:1111/xmlui/bitstream/handle/123456789/3/Su%20et%20alvScience%20Signaling%202009.pdf.
- ^ a b Wallach J.V. (January 2009). "Endogenous hallucinogens as ligands of the trace amine receptors: a possible role in sensory perception". Medical Hypotheses 72 (1): 91–4. doi:10.1016/j.mehy.2008.07.052. PMID 18805646.
- ^ Jacob M.S., Presti D.E. (2005). "Endogenous psychoactive tryptamines reconsidered: an anxiolytic role for dimethyltryptamine" (PDF). Medical Hypotheses 64 (5): 930–7. doi:10.1016/j.mehy.2004.11.005. PMID 15780487. http://crfdl.org:1111/xmlui/bitstream/handle/123456789/381/Jacob%20&%20Presti.%202004.%20Endogenous%20Tryptamines.pdf.
- ^ Morinan A., Collier J.G. (1981). "Effects of pargyline and SKF-525A on brain N,N-dimethyltryptamine concentrations and hyperactivity in mice". Psychopharmacology 75 (2): 179–83. doi:10.1007/BF00432184. PMID 6798607.
- ^ Bruns D., Riedel D., Klingauf J., Jahn R. (October 2000). "Quantal release of serotonin". Neuron 28 (1): 205–20. doi:10.1016/S0896-6273(00)00097-0. PMID 11086995.
- ^ a b c Torres, Constantino Manuel; Repke, David B. (2006). Anadenanthera: Visionary Plant Of Ancient South America. Binghamton, NY: Haworth Herbal. pp. 107–122. ISBN 978-0789026422.
- ^ Ott J. (2001). "Pharmañopo-psychonautics: human intranasal, sublingual, intrarectal, pulmonary and oral pharmacology of bufotenine" (PDF). Journal of Psychoactive Drugs 33 (3): 273–81. doi:10.1080/02791072.2001.10400574. PMID 11718320. http://files.shroomery.org/attachments/8588382-pharmanopo_J_Ott_2001_J_Psych_Drug.pdf.
- ^ Haroz, R; Greenberg, M (2005). "Emerging Drugs of Abuse". Medical Clinics of North America 89 (6): 1259–76. doi:10.1016/j.mcna.2005.06.008. PMID 16227062.
- ^ Callaway J.C., Grob C.S. (1998). "Ayahuasca preparations and serotonin reuptake inhibitors: a potential combination for severe adverse interactions" (PDF). Journal of Psychoactive Drugs 30 (4): 367–9. doi:10.1080/02791072.1998.10399712. PMID 9924842. http://wiki.dmt-nexus.com/w/images/a/a0/Ayahuasca_and_SSRI_Interactions.pdf.
- ^ "MAOIs and Diet". http://www.mayoclinic.com. http://www.mayoclinic.com/health/maois/HQ01575. Retrieved 2009-02-22.
- ^ "2C-B, DMT, You and Me". Maps. http://www.maps.org/news-letters/v12n1/12125set.html. Retrieved 2007-01-13.
- ^ "Entheogens & Visionary Medicine Pages". Miqel.com. http://www.miqel.com/entheogens/psychedelics_entheogens.html. Retrieved 2007-08-17.
- ^ Erowid (14 February 1999). "5-MeO-DMT dosage". Erowid 5-MeO-DMT Vault. http://www.erowid.org/chemicals/5meo_dmt/5meo_dmt_dose.shtml. Retrieved 8 December 2010.
- ^ Callaway JC, Raymon LP, Hearn WL, et al. Quantitation of N,N-dimethyltryptamine and harmala alkaloids in human plasma after oral dosing with ayahuasca (1996). "Quantitation of N,N-dimethyltryptamine and harmala alkaloids in human plasma after oral dosing with ayahuasca". J. Anal. Toxicol. 20 (6): 492–497. PMID 8889686.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 9th edition, Biomedical Publications, Seal Beach, CA, 2011, pp. 525-526.
- ^ Gracie, Zarkov (August 1985). "DMT - How and why to get off ...a note from underground - Number 3". Erowid DMT Vault. http://www.erowid.org/chemicals/dmt/dmt_info1.shtml. Retrieved 8 December 2010.
- ^ 2001 Strassman, Rick. DMT: The Spirit Molecule. Park Street Press, Vermont.
- ^ http://www.npr.org/templates/story/story.php?storyId=104240746&sc=fb&cc=fp
- ^ Callaway J (1988). "A proposed mechanism for the visions of dream sleep". Med Hypotheses 26 (2): 119–24. doi:10.1016/0306-9877(88)90064-3. PMID 3412201.
- ^ Hancock, Graham (2005). Supernatural: Meetings with the Ancient Teachers of Mankind. London: Century. ISBN 978-1844136810.
- ^ Hoffer A., Osmond H., Smythies J. (January 1954). "Schizophrenia; a new approach. II. Result of a year's research". Journal of Mental Science 100 (418): 29–45. doi:10.1192/bjp.100.418.29. PMID 13152519.
- ^ Schaepe, Herbert (2001). "International control of the preparation "ayahuasca"" (JPG). Erowid. http://www.erowid.org/chemicals/ayahuasca/images/archive/ayahuasca_law_undcp_fax1.jpg. Retrieved November 29, 2010.
- ^ DMT itself is already controlled under current laws. The changes include other similar blanket bans for other substances such as a ban on any and all plants containing Mescaline or Ephedrine. "Consultation on implementation of model drug schedules for Commonwealth serious drug offences". Australian Government, Attorney-General’s Department. 24 June 2010. http://www.ag.gov.au/www/agd/agd.nsf/Page/Consultationsreformsandreviews_ConsultationonimplementationofmodeldrugschedulesforCommonwealthseriousdrugoffences.
- ^ Stuff.co.nz. "New Zealand Drug - Class A DMT". http://www.stuff.co.nz/marlborough-express/news/5025678/Rare-drug-bound-for-Blenheim.
- ^ Church of the Holy Light of the Queen v. Mukasey
- ^ Church of the Holy Light of the Queen v. Mukasey (D. Ore. 2009) (“permanently enjoins Defendants from prohibiting or penalizing the sacramental use of Daime tea by Plaintiffs during Plaintiffs' religious ceremonies”). Text
External links
- Erowid DMT Vault
- TheSite.org entry on DMT
- DMT chapter from TiHKAL
- DMT: The Spirit Molecule, an overview by its author, Rick Strassman
- DMT-Nexus, a DMT users' community portal
Neurotransmitters Amino acids Alanine · Aspartate · Cycloserine · DMG · GABA · Glutamate · Glycine · Hypotaurine · Kynurenic acid (Transtorine) · NAAG (Spaglumic acid) · NMG (Sarcosine) · Serine · Taurine · TMG (Betaine)
Endocannabinoids 2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide · PEA (Palmitoylethanolamide) · RVD-Hpα · Hp (Hemopressin)
Gasotransmitters Monoamines Purines Trace amines 3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
Others 1,4-BD · Acetylcholine · GBL · GHB · Histamine
See also Template:NeuropeptidesHallucinogens Psychedelics
5-HT2AR agonists- Lysergamides: AL-LAD
- ALD-52
- BU-LAD
- CYP-LAD
- DAM-57
- Diallyllysergamide
- Ergometrine (Ergonovine, Ergobasine)
- ETH-LAD
- LAE-32
- LSA (Ergine, Lysergamide)
- LSD
- LSH
- LPD-824
- LSM-775
- Lysergic Acid 2-Butyl Amide
- Lysergic Acid 2,4-Dimethylazetidide
- Lysergic Acid 3-Pentyl Amide
- Methylergometrine
- Methylisopropyllysergamide
- Methysergide
- MLD-41
- PARGY-LAD
- PRO-LAD;
- Phenethylamines: Aleph
- 2C-B
- 2C-B-Dragonfly
- 2C-B-FLY
- 2C-C-FLY
- 2C-D-FLY
- 2C-E-FLY
- 2C-I-FLY
- 2CBFly-NBOMe
- 2C-T-7-FLY
- 2C-C
- 2C-C-NBOMe
- 2C-CN-NBOMe
- 2C-D
- 2CD-5EtO
- 2C-D-NBOMe
- 2C-E
- 2C-EF
- 2C-E-NBOMe
- 2C-F
- 2C-F-NBOMe
- 2C-G
- 2C-G-NBOMe
- 2C-H-NBOMe
- 2C-I
- 2C-N
- 2C-N-NBOMe
- 2C-O
- 2C-O-4
- 2C-P
- 2C-T
- 2C-T-2
- 2C-T-4
- 2C-T-4-NBOMe
- 2C-T-7
- 2C-T-7-NBOH
- 2C-T-8
- 2C-T-9
- 2C-T-13
- 2C-T-15
- 2C-T-17
- 2C-T-21
- 2C-TFM
- 2C-TFM-NBOMe
- 2C-YN
- 2CBCB-NBOMe
- 25B-NBOMe
- 25I-NBMD
- 25I-NBOH
- 25I-NBOMe
- 3C-E
- 3C-P
- 5-APB
- 5-APDB
- 6-APB
- 6-APDB
- Br-DFLY
- DESOXY
- DMMDA
- DMMDA-2
- DOB
- DOB-FLY
- DOM-FLY
- DOC
- DOEF
- DOET
- DOF
- DOI
- DOM
- DON
- DOPR
- DOTFM
- Escaline
- Ganesha
- HOT-2
- HOT-7
- HOT-17
- IAP
- Isoproscaline
- Jimscaline
- Lophophine
- MDA
- MDEA
- MDMA
- MMA
- MMDA
- MMDA-2
- MMDA-3a
- MMDMA
- Macromerine
- Mescaline
- Methallylescaline
- NBOMe-mescaline
- Proscaline
- TCB-2
- TFMFly
- TMA;
- Piperazines: pFPP
- TMFPP;
- Tryptamines: 1-Methyl-5-methoxy-diisopropyltryptamine
- 2,N,N-TMT
- 4-HO-5-MeO-DMT
- 4-Acetoxy-DET
- 4-Acetoxy-DIPT
- 4-Acetoxy-DMT
- 4-Acetoxy-DPT
- 4-Acetoxy-MiPT
- 4-HO-DPT
- 4-HO-MET
- 4-Propionyloxy-DMT
- 4-HO-MPMI
- 5-Me-MIPT
- 5-N,N-TMT
- 5-AcO-DMT
- 5-MeO-2,N,N-TMT
- 5-MeO-α,N,N-TMT
- 5-MeO-α-ET
- 5-MeO-α-MT
- 5-MeO-DALT
- 5-MeO-DET
- 5-MeO-DIPT
- 5-MeO-DMT
- 5-MeO-DPT
- 5-MeO-EiPT
- 5-MeO-MET
- 5-MeO-MIPT
- 5-MeO-MPMI
- 7,N,N-TMT
- α,N,N-TMT
- α-ET
- α-MT
- AL-37350A
- Baeocystin
- Bufotenin
- DALT
- DBT
- DCPT
- DET
- DIPT
- DMT
- DPT
- EiPT
- Ethocin
- Ethocybin
- Iprocin
- MET
- Miprocin
- MIPT
- Norbaeocystin
- PiPT
- Psilocin
- Psilocybin;
- Others: AL-38022A
- Ibogaine
- Noribogaine
- Voacangine
Dissociatives
NMDAR antagonists- Arylcyclohexylamines: 3-MeO-PCP
- 4-MeO-PCP
- Dieticyclidine
- Esketamine
- Eticyclidine
- Gacyclidine
- Ketamine
- Methoxetamine
- Neramexane
- Phencyclidine
- PCPr
- Rolicyclidine
- Tenocyclidine
- Tiletamine;
- Morphinans: Dextrallorphan
- Dextromethorphan
- Dextrorphan
- Methorphan (Racemethorphan)
- Racemorphan;
- Others: 2-MDP
- 8A-PDHQ
- Aptiganel
- Dexoxadrol
- Dizocilpine (MK-801)
- Etoxadrol
- Ibogaine
- Midafotel
- NEFA
- Nitrous Oxide
- Noribogaine
- Perzinfotel
- Remacemide
- Selfotel
- Xenon
Deliriants
mAChR antagonists- 3-Quinuclidinyl benzilate
- Atropine
- Benactyzine
- Benzatropine
- Benzydamine
- Biperiden
- Brompheniramine
- CAR-226,086
- CAR-301,060
- CAR-302,196
- CAR-302,282
- CAR-302,368
- CAR-302,537
- CAR-302,668
- Chlorpheniramine
- Chloropyramine
- Clemastine
- CS-27349
- Cyclizine
- Cyproheptadine
- Dicyclomine (Dicycloverine)
- Dimenhydrinate
- Diphenhydramine
- Ditran
- Doxylamine
- EA-3167
- EA-3443
- EA-3580
- EA-3834
- Elemicin
- Flavoxate
- Hydroxyzine
- Hyoscyamine
- Meclizine
- Myristicin
- N-Ethyl-3-piperidyl benzilate
- N-Methyl-3-piperidyl benzilate
- Pyrilamine
- Orphenadrine
- Oxybutynin
- Pheniramine
- Phenyltoloxamine
- Procyclidine
- Promethazine
- Scopolamine (Hyoscine)
- Tolterodine
- Trihexyphenidyl
- Tripelennamine
- Triprolidine
- WIN-2299
Miscellaneous - Apomorphine
- Bromocriptine
- Cabergoline
- Lisuride
- Memantine
- Pergolide
- Piribedil
- Pramipexole
- Ropinirole
- Rotigotine
- Butane
- Chloroform
- Diethyl Ether (Ether)
- Enflurane
- Freon
- Gasoline (Petrol)
- Kerosene (Paraffin)
- Propane
- Toluene
κOR agonists- 2-EMSB
- 2-MMSB
- Alazocine
- Bremazocine
- Butorphanol
- Cyclazocine
- Cyprenorphine
- Dextrallorphan
- Dezocine
- Enadoline
- Herkinorin
- HZ-2
- Ibogaine
- Ketazocine
- Metazocine
- Nalbuphine
- Nalorphine
- Noribogaine
- Pentazocine
- Phenazocine
- Salvinorin A
- Spiradoline
- Tifluadom
- U-50,488
- U-69,593
- Dextrallorphan
- Dextromethorphan
- Dextrorphan
- Noscapine (Narcotine)
OthersAdrenergics Receptor ligands Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Cannabivarin • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.βAgonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • XipranololReuptake inhibitors Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • melitracen • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Ginkgo biloba • Indeloxazine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Tedatioxetine • Teniloxazine • Tramadol • ZiprasidoneReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-OH-PEA • 4-CAB • 4-FA • 4-FMA • 4-MA • 4-MMA • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Me-PEA • BDB • Benzphetamine • BOH • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • DMA • DMMA • EBDB • Ephedrine • Ethcathinone • Ethylamphetamine • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • IAP • IMP • L-Deprenyl (Selegiline) • Lisdexamfetamine • Lophophine • MBDB • MDA (Tenamfetamine) • MDEA • MDMA • MDMPEA • MDOH • MDPEA • Mefenorex • Mephedrone • Mephentermine • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methcathinone • Methedrone • Methylone • NAP • Ortetamine • Paredrine • pBA • pCA • Pentorex (Phenpentermine) • Phenethylamine • Pholedrine • Phenpromethamine • Phentermine • Phenylpropanolamine • pIA • Prenylamine • Propylamphetamine • Pseudoephedrine • Tiflorex • Tyramine • Xylopropamine • Zylofuramine; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • pFPP; Others: 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-Benzylpiperidine • 4-Benzylpiperidine • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanorex • Isometheptene • Methylhexanamine • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • TuaminoheptaneEnzyme inhibitors 3,4-DihydroxystyreneDBHCGS-19281A • SKF-64139 • SKF-7698Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline • Safinamide
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.COMTOthers Ferrous Iron (Fe2+) • S-Adenosyl-L-Methionine • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal Phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)List of adrenergic drugsDopaminergics Receptor ligands AgonistsAdamantanes: Amantadine • Memantine • Rimantadine; Aminotetralins: 7-OH-DPAT • 8-OH-PBZI • Rotigotine • UH-232; Benzazepines: 6-Br-APB • Fenoldopam • SKF-38,393 • SKF-77,434 • SKF-81,297 • SKF-82,958 • SKF-83,959; Ergolines: Bromocriptine • Cabergoline • Dihydroergocryptine • Lisuride • LSD • Pergolide; Dihydrexidine derivatives: 2-OH-NPA • A-86,929 • Ciladopa • Dihydrexidine • Dinapsoline • Dinoxyline • Doxanthrine; Others: A-68,930 • A-77,636 • A-412,997 • ABT-670 • ABT-724 • Aplindore • Apomorphine • Aripiprazole • Bifeprunox • BP-897 • CY-208,243 • Dizocilpine • Etilevodopa • Flibanserin • Ketamine • Melevodopa • Modafinil • Pardoprunox • Phencyclidine • PD-128,907 • PD-168,077 • PF-219,061 • Piribedil • Pramipexole • Propylnorapomorphine • Pukateine • Quinagolide • Quinelorane • Quinpirole • RDS-127 • Ro10-5824 • Ropinirole • Rotigotine • Roxindole • Salvinorin A • SKF-89,145 • Sumanirole • Terguride • Umespirone • WAY-100,635AntagonistsTypical antipsychotics: Acepromazine • Azaperone • Benperidol • Bromperidol • Clopenthixol • Chlorpromazine • Chlorprothixene • Droperidol • Flupentixol • Fluphenazine • Fluspirilene • Haloperidol • Loxapine • Mesoridazine • Methotrimeprazine • Nemonapride • Penfluridol • Perazine • Periciazine • Perphenazine • Pimozide • Prochlorperazine • Promazine • Sulforidazine • Sulpiride • Sultopride • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Trifluperidol • Zuclopenthixol; Atypical antipsychotics: Amisulpride • Asenapine • Blonanserin • Cariprazine • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Lurasidone • Melperone • Molindone • Mosapramine • Olanzapine • Paliperidone • Perospirone • Piquindone • Quetiapine • Remoxipride • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Antiemetics: AS-8112 • Alizapride • Bromopride • Clebopride • Domperidone • Metoclopramide • Thiethylperazine; Others: Amoxapine • Buspirone • Butaclamol • Ecopipam • EEDQ • Eticlopride • Fananserin • L-745,870 • Nafadotride • Nuciferine • PNU-99,194 • Raclopride • Sarizotan • SB-277,011-A • SCH-23,390 • SKF-83,959 • Sonepiprazole • Spiperone • Spiroxatrine • Stepholidine • Tetrahydropalmatine • Tiapride • UH-232 • YohimbineReuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • Tedatioxetine • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugsSerotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969 • Vortioxetine
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507 • VortioxetineReuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DCA • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersSigmaergics Receptor
Ligands3-PPP • 4-IBP • 4-PPBP • Afobazole • Allylnormetazocine • Amitriptyline • Amphetamine • Berberine • Citalopram • Clorgyline • Cocaine • Cyclazocine • Dextrallorphan • Dextromethorphan • Dextrorphan • DTG • BD-1,008 • Desipramine • DHEA • Dimethyltryptamine • Dimemorfan • Ditolylguanidine • EMD-57,445 • Escitalopram • Fluoxetine • Fluvoxamine • Heroin • Igmesine • Imipramine • JO-1,784 • L-687,384 • Lamotrigine • Lu 28-179 • MDMA • Methamphetamine • Morphine • Noscapine • OPC-14,523 • Opipramol • PB-28 • PD-144,415 • Pentazocine • Pentoxyverine • Phencyclidine • PRE-084 • Pregnenolone • RTI-55 • SA-4503 • Sertraline • Siramesine • VenlafaxineBD-1,047 • BD-1,063 • BMY-14,802 • E-5,842 • Haloperidol • NE-100 • Progesterone • Rimcazole • SM-21UnknownClocapramine • GevotrolineTryptamines 1-(2-Dimethylaminoethyl)dihydropyrano(3,2-e)indole • 2-Methyl-5-HT • 4-Acetoxy-DET • 4-Acetoxy-DIPT • 4-Acetoxy-DMT • 4-HO-αMT • 4-HO-DIPT • 4-HO-MET • 4-MeO-DMT • 4-Methyl-αET • 4-Methyl-αMT • 5-Benzyloxytryptamine • 5-Bromo-DMT • 5-Carboxamidotryptamine • 5-Ethoxy-αMT • 5-Fluoro-αMT • 5-HO-αMT • 5-HTP • 5-Ethoxy-DMT • 5-Ethyl-DMT • 5-Fluoro-DMT • 5-Methyl-DMT • 5-Methoxytryptamine • 5-MeO-7,N,N-TMT • 5-Methyl-αET • 5-MeO-αET • 5-MeO-αMT • 5-MeO-DALT • 5-MeO-DET • 5-MeO-DIPT • 5-MeO-DMT • 5-MeO-DPT • 5-MeO-MALT • 5-MeO-MIPT • 5,7-Dihydroxytryptamine • 5-(Nonyloxy)tryptamine • 6-Fluoro-αMT • 7-Methyl-αET • 7-Methyl-DMT • αET • αMT • Aeruginascin • AL-37350A • BW-723C86 • Baeocystin • Bufotenidine • Bufotenin • DALT • Desformylflustrabromine • DET • DiPT • DMT • DPT • Ethocybin • EiPT • EMDT • EPT • Ethocin • FGIN-127 • FGIN-143 • Ibogaine • Indorenate • Iprocin • MET • MiPT • Miprocin • Melatonin • MS-245 • NAS • NMT • Norbaeocystin • Normelatonin • Oxypertine • PiPT • Psilocin • Psilocybin • Rizatriptan • Serotonin • Sumatriptan • Tryptamine • Tryptophan • Yohimbine • Yuremamine • Zolmitriptan
Drugs from TiHKAL AL-LAD • DBT • DET • DiPT • 5-MeO-α-MT • DMT • 2,α-DMT • α,N-DMT • DPT • EiPT • α-ET • ETH-LAD • Harmaline • Harmine • 4-HO-DBT • 4-HO-DET • 4-HO-DiPT • 4-HO-DMT • 5-HO-DMT • 4-HO-DPT • 4-HO-MET • 4-HO-MiPT • 4-HO-MPT • 4-HO-pyr-T • Ibogaine • LSD • MBT • 4,5-MDO-DiPT • 5,6-MDO-DiPT • 4,5-MDO-DMT • 5,6-MDO-DMT • 5,6-MDO-MiPT • 2-Me-DET • 2-Me-DMT • Melatonin • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 4-MeO-MiPT • 5-MeO-MiPT • 5,6-MeO-MiPT • 5-MeO-NMT • 5-MeO-pyr-T • 6-MeO-THH • 5-MeO-TMT • 5-MeS-DMT • MiPT • α-MT • NET • NMT • PRO-LAD • pyr-T • Tryptamine • Tetrahydroharmine • α,N,O-TMS
Categories:- Ayahuasca
- Entheogens
- Psychedelic tryptamines
- Natural tryptamine alkaloids
- Serotonin receptor agonists
Wikimedia Foundation. 2010.



