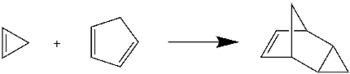- Cyclopropene
-
Cyclopropene 

 Systematic nameCyclopropene[1]
Systematic nameCyclopropene[1]Identifiers CAS number 2781-85-3 
PubChem 123173 ChemSpider 109788 
MeSH cyclopropene Jmol-3D images Image 1 - C1C=C1
Properties Molecular formula C3H4 Molar mass 40.06 g mol−1 Exact mass 40.031300128 g mol-1 Boiling point -36 °C, 237 K, -33 °F
Thermochemistry Std enthalpy of
combustion ΔcHo298-2032--2026 kJ mol-1 Specific heat capacity, C 51.9-53.9 J K-1 mol-1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cyclopropene is an organic compound with the formula C3H4. It is the simplest isolable cycloalkene. It has a triangular structure. Because the ring is highly strained, cyclopropene is both difficult to prepare and interesting to study.[2] Like cyclopropane, the carbon ring of cyclopropene is planar. The reduced length of the double bond bond compared to an single bond causes the angle opposite the double bond to narrow to about 51°[3] from the 60° angle found in cyclopropane. As with cyclopropane, the carbon–carbon bonding in the ring has increased p character: the alkene carbons use sp2.68 hybridization for the ring.[4]
Contents
Synthesis of cyclopropene and derivatives
Early syntheses
The first confirmed synthesis of cyclopropene, carried out by Dem'yanov and Doyarenko, involved the thermal decomposition of trimethylcyclopropylammonium hydroxide over platinized clay at 320–330 °C under a CO2 atmosphere. This reaction produces mainly trimethylamine and dimethylcyclopropyl amine, together with about 5% of cyclopropene. Cyclopropene can also be obtained in about 1% yield by thermolysis of the adduct of cycloheptatriene and dimethyl acetylenedicarboxylate.
Modern syntheses from allyl chlorides
Allyl chloride undergoes dehydrohalogenation upon treatment with the base sodium amide at 80 °C to produce cyclopropene in about 10% yield.[5]
-
- CH2=CHCH2Cl + NaNH2 → C3H4 (cyclopropene) + NaCl + NH3
The major byproduct of the reaction is allyl amine. Adding allyl chloride to sodium bis(trimethylsilyl)amide in boiling toluene over a period of 45–60 minutes produces the targeted compound in about 40% yield with an improvement in purity:[6]
-
- CH2=CHCH2Cl + NaN(TMS)2 → C3H4 (cyclopropene) + NaCl + NH(TMS)2
1-Methylcyclopropene is synthesized similarly but at room temperature from methallylchloride using phenyllithium as the base:[7]
-
- CH2=C(CH3)CH2Cl + LiC6H5 → CH3C3H3 (1-methylcylopropene) + LiCl + C6H6
Syntheses of derivatives
Treatment of nitrocyclopropanes with sodium methoxide eliminates the nitrite, giving the respective cyclopropene derivative. The synthesis of purely aliphatic cyclopropenes was first illustrated by the copper-catalyzed additions of carbenes to alkynes. In the presence of a copper sulfate catalyst, ethyl diazoacetate reacts with acetylenes to give cyclopropenes. 1,2-Dimethylcyclopropene-3-carboxylate arises via this method from 2-butyne. Copper has proved to be useful as a catalyst in a variety of cyclopropene syntheses. Copper sulfate and copper dust are among the more popular forms of copper used.
Reactions of cyclopropene
Studies on cyclopropene mainly focus on the consequences of its high ring strain. At 425 °C, cyclopropene isomerizes to methylacetylene (propyne).
- C3H4 → H3CC≡CH
Attempted fractional distillation of cyclopropene at –36 °C (its predicted boiling point) results in polymerization. The mechanism is assumed to be a free-radical chain reaction, and the product, based on NMR spectra, is thought to be polycyclopropane.
Cyclopropene undergoes the Diels–Alder reaction with cyclopentadiene to give endo-tricyclo[3.2.1.02,4]oct-6-ene. This reaction is commonly used to check for the presence of cyclopropene, following its synthesis.[6]
Related compounds
- Malvalic acid is a toxic cyclopropene fatty acid that occurs in cottonseed oil.
- 1-Methylcyclopropene (1-MCP) is used to slow the ripening in fruits.[8][9]
- Borirenes, phosphirenes, and silirenes are boron-, phosphorus-, and silicon-substituted cyclopropenes, with the formula RBC2R'2, RPC2R'2, and R2SiC2R'2.
External links
References
- ^ "cyclopropene - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identification and Related Records. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=123173&loc=ec_rcs. Retrieved 9 October 2011.
- ^ Carter, F. L.; Frampton, V. L. (1964). "Review of the Chemistry of Cyclopropene Compounds". Chemical Reviews 64: 497–525. doi:10.1021/cr60231a001.
- ^ Staley, S. W.; Norden, T. D.; Su, C.-F.; Rall, M.; Harmony, M. D. (1987). "Structure of 3-cyanocyclopropene by microwave spectroscopy and ab initio molecular orbital calculations. Evidence for substituent-ring double bond interactions". J. Am. Chem. Soc. 109 (10): 2880–2884. doi:10.1021/ja00244a004.
- ^ Allen, F. H. (1982). "The geometry of small rings: Molecular geometry of cyclopropene and its derivatives". Tetrahedron 38 (5): 645–655. doi:10.1016/0040-4020(82)80206-8.
- ^ Closs, G.L.; Krantz, K.D. (1966). "A Simple Synthesis of Cyclopropene". Journal of Organic Chemistry 31: 638. doi:10.1021/jo01340a534.
- ^ a b Binger, P.; Wedermann, P.; Brinker, U. H. (2000), "Cyclopropene: A New Simple Synthesis and Its Diels-Alder reaction with Cyclopentadiene", Org. Synth. 77: 254, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=v77p0254; Coll. Vol. 10: 231
- ^ Clarke, T. C.; Duncan, C. D.; Magid, R. M. (1971). "An Efficient and Convenient Synthesis of 1-Methylcyclopropene". J. Org. Chem 36: 1320. doi:10.1021/jo00808a041.
- ^ Beaudry, R.; Watkins, C. (2001). "Use of 1-MCP on Apples". Perishable Handling Quarterly (University of California) (108): 12.
- ^ Trinchero, G. D.; Sozzi, G. O.; Covatta, F.; Fraschina, A. A. (May 2004). "Inhibition of ethylene action by 1-methylcyclopropene extends postharvest life of "Bartlett" pears". Postharvest Biology and Technology 32 (2): 193–204. doi:10.1016/j.postharvbio.2003.11.009.
Alkenes Dienes Cyclobutadiene · Cyclopentadiene · Cyclohexadiene (1,3-Cyclohexadiene · 1,4-Cyclohexadiene) · Cycloheptadiene · Cyclooctadiene (1,5-Cyclooctadiene)Categories:- Cycloalkenes
Wikimedia Foundation. 2010.