- Ketoconazole
-
Ketoconazole 

Systematic (IUPAC) name 1-[4-(4-{[(2R,4S)-2-(2,4-Dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazin-1-yl]ethan-1-one Clinical data Trade names Nizoral AHFS/Drugs.com monograph MedlinePlus a682816 Licence data US FDA:link Pregnancy cat. B3(AU) C(US) Legal status POM (UK) OTC (US) Routes Oral, topical Pharmacokinetic data Bioavailability Variable Protein binding 84 to 99% Metabolism Hepatic Half-life Biphasic: Excretion Biliary and renal Identifiers CAS number 65277-42-1 
ATC code J02AB02 D01AC08 G01AF11 PubChem CID 456201 DrugBank APRD00401 ChemSpider 401695 
UNII R9400W927I 
KEGG D00351 
ChEBI CHEBI:48336 
ChEMBL CHEMBL75 
Chemical data Formula C26H28Cl2N4O4 Mol. mass 531.431 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Ketoconazole (
 /ˌkiːtɵˈkoʊnəzɒl/) is a synthetic antifungal drug used to prevent and treat fungal skin infections, especially in immunocompromised patients such as those with AIDS or those on chemotherapy. Ketoconazole is sold commercially as an anti-dandruff shampoo, topical cream, and oral tablet.
/ˌkiːtɵˈkoʊnəzɒl/) is a synthetic antifungal drug used to prevent and treat fungal skin infections, especially in immunocompromised patients such as those with AIDS or those on chemotherapy. Ketoconazole is sold commercially as an anti-dandruff shampoo, topical cream, and oral tablet.Ketoconazole is very lipophilic, which leads to accumulation in fatty tissues. The less toxic and more effective triazole compounds fluconazole and itraconazole have largely replaced ketoconazole for internal use.[citation needed] Ketoconazole is best absorbed at highly acidic levels, so antacids or other causes of decreased stomach acid levels will lower the drug's absorption when taken orally. Absorption can be increased by taking it with an acidic beverage, such as cola.[1]
Contents
Medical uses
Antifungal
Ketoconazole is usually prescribed for topical infections such as athlete's foot, ringworm, candidiasis (yeast infection or thrush), and jock itch. The over-the-counter shampoo version can also be used as a body wash for the treatment of tinea versicolor.[2][3] Ketoconazole is used to treat eumycetoma, the fungal form of mycetoma.
The side effects of ketoconazole are sometimes used to treat non-fungal problems. The decrease in testosterone caused by the drug makes it useful for treating prostate cancer and for preventing post-operative erections[4] following penile surgery. Another use is the suppression of glucocorticoid synthesis, where it is used in the treatment of Cushing's syndrome.[5] These side effects have also been studied for use in reducing depressive symptoms [6] and drug addiction;[7] however, it has not succeeded in either of these roles.[8][9]
Ketoconazole is also used in combination with other drugs such as zinc pyrithione in rinse-off products. The anti-dandruff shampoo is designed for people who have a more serious case of dandruff where symptoms include, but are not limited to constant non-stop flaking, and severe itchiness.
It is a pregnancy category C drug because animal testing has shown it to cause teratogenesis in high dosages. Until recently, there were two human test cases on record (both during the treatment of Cushing's syndrome)[10][11] and no adverse effects were reported, but this is not a broad enough data sample to draw any meaningful conclusions. A subsequent trial in Europe failed to show a risk to infants of mothers receiving ketoconazole.[12]
Hair loss
Some preliminary research suggests that ketoconazole shampoo may be beneficial in men suffering from androgenic alopecia. Support for this comes primarily from a single study in 1998 that compared ketoconazole to the proven hair loss drug minoxidil in men with androgenic alopecia. [13] The FDA do not accept that there is enough evidence to market it for hair loss.[14]
Veterinary
This medication is also sometimes prescribed by veterinarians for use on pets, often as unflavored tablets that may need to be cut to smaller size for correct dosage.[15]
Mechanism of action
As an antifungal, ketoconazole is structurally similar to imidazole and interferes with the fungal synthesis of ergosterol, a constituent of fungal cell membranes, as well as certain enzymes. As with all azole antifungal agents, ketoconazole works principally by inhibiting the enzyme cytochrome P450 14-alpha-demethylase (P45014DM). This enzyme participates in the sterol biosynthesis pathway that leads from lanosterol to ergosterol. Lower doses of fluconazole and itraconazole are required to kill fungi compared to ketoconazole, as they have been found to have a greater affinity for fungal cell membranes.
As an antiandrogen, ketoconazole operates through at least two mechanisms of action. First, and most notably, high oral doses of ketoconazole (e.g. 400 mg 3x/day) block both testicular and adrenal androgen biosynthesis, leading to a reduction in circulating testosterone levels.[16] Ketoconazole produces this effect through inhibition of cytochrome P450 and 17,20-lyase, which are involved in the synthesis and degradation of steroids, including the precursors of testosterone. Due to its efficacy at reducing systemic androgen levels, ketoconazole has been used as a treatment for androgen-dependent prostate cancer.[17] Second, ketoconazole is an androgen receptor antagonist, competing with androgens such as testosterone and DHT for androgen receptor binding. This effect is thought to be quite weak, even with high oral doses of ketoconazole.[18]
Susceptible fungi
Ketoconazole inhibits growth of dermatophytes and yeast species such as Candida albicans. The rise in the number of HIV/AIDS immune compromised patients has led to an increase in the frequency and significance of opportunistic fungal infections. Resistance to ketoconazole has been observed in a number of clinical fungal isolates, including C. albicans. Experimentally resistance usually arises as a result of mutations in the sterol biosynthesis pathway. Defects in the sterol 5-6 desaturase enzyme reduce the toxic effects of azole inhibition of the 14-alpha demethylation step. Multidrug-Resistance Genes (MDR)[19] can also play a role in reducing cellular levels of the drug. As azole antifungals all act at the same point in the sterol pathway, resistant isolates are normally cross-resistant to all members of the azole family.
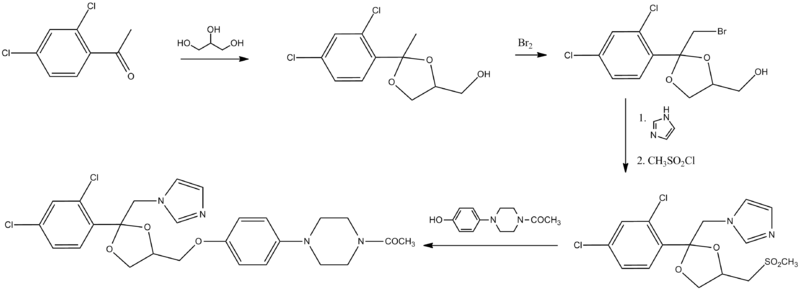
Synthesis
The chemical synthesis of ketoconazole begins with dichloroacetophenone:[20]
History
Ketoconazole was discovered in 1976 and released in 1981.[21] It followed griseofulvin as one of the first available oral treatments for fungal infections.
Brand names
It is marketed under the trademark name Nizoral by Ortho-McNeil Pharmaceutical in the United States, Australia and Canada, as Sebizole by Douglas Pharmaceuticals in Australia and New Zealand and as Ketomed in Latin America.[22] In Spain, products with ketoconazole as main agent include Ketoisdin gel (gel) and Fungarest (cream). In India, ketoconazole is sold as keton (tablets,soap & cream) by Green Apple Lifesciences Limited.
References
- ^ T W Chin, M Loeb, and I W Fong (August 1995). "Effects of an acidic beverage (Coca-Cola) on absorption of ketoconazole.". Antimicrobial agents and chemotherapy 39 (8): 1671–5. PMC 162805. PMID 7486898. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=162805.
- ^ MedlinePlus Medical Encyclopedia: Tinea versicolor
- ^ Tinea Versicolor
- ^ Evans, K. C.; A. C. Peterson, H. E. Ruiz and R. A. Costabile (August 2004). "Use of oral ketoconazole to prevent postoperative erections following penile surgery". International Journal of Impotence Research 16 (4): 346–349. doi:10.1038/sj.ijir.3901160. PMID 14973533. http://www.nature.com/ijir/journal/v16/n4/full/3901160a.html.
- ^ Loli, Paola; Maria Elisa Berselli and Mariantonella Tagliaferri (1986). "Use of ketoconazole in the treatment of Cushing's syndrome". Journal of Clinical Endocrinology & Metabolism 63 (6): 1365–71. doi:10.1210/jcem-63-6-1365. PMID 3023421.
- ^ Wolkowitz, Owen M.; Victor I. Reus (September 1999). "Treatment of depression with antiglucocorticoid drugs". Psychosomatic Medicine 61 (5): 698–711. PMID 10511017. http://www.psychosomaticmedicine.org/cgi/content/full/61/5/698.
- ^ Goeders, Nick E.; Rachel L. Peltiera and Glenn F. Guerin (December 1998). "Ketoconazole reduces low dose cocaine self-administration in rats". Drug and Alcohol Dependence 53 (1): 67–77. doi:10.1016/S0376-8716(98)00108-2. PMID 10933341.
- ^ Malison, Robert T.; Amit Anand, Gregory H. Pelton, Paul Kirwin, Linda Carpenter, Christopher J. McDougle, George R. Heninger and Lawrence H. Price (October 1999). "Limited efficacy of ketoconazole in treatment-refractory major depression". Journal of Clinical Psychopharmacology 19 (5): 466–470. doi:10.1097/00004714-199910000-00011. PMID 10505589.
- ^ Ward, Amie S.; Eric D. Collins, Margaret Haney, Richard W. Foltin and Marian W Fischman (November 1998). "Ketoconazole attenuates the cortisol response but not the subjective effects of smoked cocaine in humans". Behavioural Pharmacology 9 (7): 577–86. doi:10.1097/00008877-199811000-00013. PMID 9862083.
- ^ Amado, José Antonio; Carlos Pesquera, Eva M. Gonzalez, Macarena Otero, Julio Freijanes, and Angel Alvarez (March 1990). "Successful treatment with ketoconazole of Cushing's syndrome in pregnancy". Postgraduate Medical Journal 66 (773): 221–3. doi:10.1136/pgmj.66.773.221. PMC 2429473. PMID 2362890. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2429473.
- ^ Berwaerts, Joris; Johan Verhelst, Charles Mahler and Roger Abs (June 1999). "Cushing's syndrome in pregnancy treated by ketoconazole: case report and review of the literature". Gynecological Endocrinology 13 (3): 175–82. doi:10.3109/09513599909167552. PMID 10451809.
- ^ Kazy, Zoltán; Erzsébet Puhó and Andrew E. Czeizel (March 2005). "Population-based case–control study of oral ketoconazole treatment for birth outcomes". Congenital Anomalies 45 (1): 5–8. doi:10.1111/j.1741-4520.2005.00053.x. PMID 15737124.
- ^ Rogers, NE; Avram, MR (2008 Oct). "Medical treatments for male and female pattern hair loss.". Journal of the American Academy of Dermatology 59 (4): 547-66; quiz 567-8. PMID 18793935.
- ^ Nizoral Shampoo as a Hair Loss Remedy?
- ^ Ketoconazole for Your Pet at Petscriptions
- ^ Witjes FJ, Debruyne FM, Fernandez del Moral P, Geboers AD (May 1989). "Ketoconazole high dose in management of hormonally pretreated patients with progressive metastatic prostate cancer. Dutch South-Eastern Urological Cooperative Group". Urology 33 (5): 411–5. PMID 2652864.
- ^ De Coster R, Wouters W, Bruynseels J (January 1996). "P450-dependent enzymes as targets for prostate cancer therapy". J. Steroid Biochem. Mol. Biol. 56 (1–6 Spec No): 133–43. doi:10.1016/0960-0760(95)00230-8. PMID 8603034.
- ^ Eil C (August 1992). "Ketoconazole binds to the human androgen receptor". Horm. Metab. Res. 24 (8): 367–70. doi:10.1055/s-2007-1003337. PMID 1526623.
- ^ MDR Gene: on Medical Dictionary Online
- ^ Heeres, J.; Backx, L. J. J.; Mostmans, J. H.; Van Cutsem, J. (1979). "Antimycotic imidazoles. Part 4. Synthesis and antifungal activity of ketoconazole, a new potent orally active broad-spectrum antifungal agent". Journal of Medicinal Chemistry 22 (8): 1003–5. doi:10.1021/jm00194a023. PMID 490531.
- ^ MedicineNet.com – ketoconazole (Nizoral, Extina, Xolegel, Kuric)
- ^ "Ketomed". Facultad de Medicina, Universidad Nacional de México. http://www.facmed.unam.mx/bmnd/plm_2k8/src/prods/46980.htm. Retrieved 2011-05-27.
Gynecological anti-infectives and antiseptics (G01) Antibiotics Arsenic compounds Quinoline derivatives Organic acids Sulfonamides SulfatolamideImidazole derivatives Metronidazole • Clotrimazole • Miconazole • Econazole • Ornidazole • Isoconazole • Tioconazole • Ketoconazole • Fenticonazole • Azanidazole • Propenidazole • Butoconazole • Omoconazole • Oxiconazole • FlutrimazoleTriazole derivatives Other Clodantoin • Inosine • Policresulen • Nifuratel • Furazolidone • Methylrosaniline • Povidone-iodine • Ciclopirox • Protiofate • Lactobacillus fermentum • Copper usnatePiperazines Simple piperazines
(no additional rings)1-Cyclohexylpiperazine • Aminoethylpiperazine • Diethylcarbamazine • HEPPS • Midafotel • Piperazine • PIPESPhenylpiperazines Acaprazine • Antrafenine • Aripiprazole • Batoprazine • Bifeprunox • BRL-15,572 • Ciprofloxacin • CSP-2503 • Dapiprazole • DCPP • DMPP • Diphenylpiperazine • Dropropizine • EGIS-12,233 • Elopiprazole • Eltoprazine • Enpiprazole • Ensaculin • Etoperidone • Flesinoxan • Flibanserin • Fluprazine • Itraconazole • Ketoconazole • Levodropropizine • Lorpiprazole • mCPP • Mefway • MeOPP • Mepiprazole • Naftopidil • Naphthylpiperazine • Nefazodone • Niaprazine • Oxypertine • Pardoprunox • pCPP • pFPP • Posaconazole • PRX-00023 • S-14,506 • S-14,671 • S-15,535 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • Sonepiprazole • TFMPP • Tolpiprazole • Trazodone • Urapidil • Vesnarinone • Vilazodone • WAY-100,135 • WAY-100,635Benzylpiperazines 2C-B-BZP • Befuraline • Bifeprunox • Buclizine • BZP • Chlorbenzoxamine • DBZP • Fipexide • Imatinib • MBZP • MDBZP • Meclozine • Piberaline • Piribedil • Trimetazidine • VesnarinoneDiphenylalkylpiperazines
(benzhydrylalkylpiperazines)Almitrine • Amperozide • BRL-15,572 • Buclizine • BW373U86 • Cetirizine • Chlorbenzoxamine • Chlorcyclizine • Cinnarizine • Clocinizine • Cyclizine • DBL-583 • Diphenylmethylpiperazine • Dotarizine • DPI-221 • DPI-287 • DPI-3290 • GBR-12,783 • GBR-12,935 • GBR-13,069 • GBR-13,098 • GBR-13,119 • Hydroxyzine • Lidoflazine • Manidipine • Meclozine • Oxatomide • SNC-80 • VanoxerinePyrimidinylpiperazines Buspirone • Dasatinib • Eptapirone • Gepirone • Ipsapirone • Piribedil • Pyrimidinylpiperazine • Revospirone • Tandospirone • Tirilazad • Trimazosin • Umespirone • ZalospironePyridinylpiperazines Atevirdine • Azaperone • PyridinylpiperazineBenzo(iso)thiazolylpiperazines Tricyclics
(piperazine attached via side chain)Others 6-Nitroquipazine • Azimilide • Cinepazet • Cyclohexylpiperazine • Hexocyclium • Indinavir • JNJ-7777120 • Lodenafil • Mirodenafil • PB-28 • Quipazine • Ranolazine • SA-4503 • Sildenafil • Tadalafil • Vardenafil • VUF-6002 • ZipeprolExternal links
- Doctor Fungus | Ketoconazole
- Ketoconazole | Patient information
- PubPK | Ketoconazole pharmacokinetics
- Janssen Pharmaceutica | Fungal infections
- Piérard-Franchimont C, Goffin V, Decroix J, Piérard GE (2002). "A multicenter randomized trial of ketoconazole 2% and zinc pyrithione 1% shampoos in severe dandruff and seborrheic dermatitis". Skin Pharmacol. Appl. Skin Physiol. 15 (6): 434–41. PMID 12476017. http://content.karger.com/produktedb/produkte.asp?typ=fulltext&file=sph15434.
Categories:- Amides
- Antiandrogens
- Antifungals
- Dioxolanes
- Endocrine disruptors
- Imidazoles
- Janssen Pharmaceutica
- Organochlorides
- Phenol ethers
- Piperazines
- Withdrawn drugs
Wikimedia Foundation. 2010.