- Pyridostigmine
-
Pyridostigmine 
Systematic (IUPAC) name 3-[(dimethylcarbamoyl)oxy]-1-methylpyridinium Clinical data Trade names Mestinon AHFS/Drugs.com monograph MedlinePlus a682229 Pregnancy cat. C(AU) C(US) Legal status POM (UK) ℞-only (US) Routes Oral, intravenous Pharmacokinetic data Bioavailability 7.6 +/- 2.4% Half-life 1.78 +/- 0.24hrs Excretion Renal Identifiers CAS number 155-97-5 
ATC code N07AA02 PubChem CID 4991 DrugBank APRD00380 ChemSpider 4817 
UNII 19QM69HH21 
KEGG D00487 
ChEMBL CHEMBL1115 
Chemical data Formula C9H13N2O2 Mol. mass 181.212 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Pyridostigmine is a parasympathomimetic and a reversible cholinesterase inhibitor. Since it is a quaternary amine, it is poorly absorbed in the gut and does not cross the blood-brain barrier, except possibly in stressful conditions.[1]
Contents
Mode of action
In a synapse, action potentials are conducted along motor nerves to their terminals where they initiate a Ca2+ influx and the release of acetylcholine (ACh). The ACh diffuses across the synaptic cleft and binds to receptors on the post synaptic membrane, causing an influx of Na+ and K+ ions, resulting in depolarization. If large enough, this depolarization results in an action potential. To prevent constant stimulation once the ACh is released, an enzyme called acetylcholinesterase is present in the endplate membrane close to the receptors on the post synaptic membrane, and quickly hydrolizes ACh.
Pyridostigmine inhibits acetylcholinesterase in the synaptic cleft, thus slowing down the hydrolysis of acetylcholine. It is a quaternary carbamate inhibitor of cholinesterase that does not cross the blood-brain barrier, and is taken daily in anticipation of an attack, which carbamylates about 30% of peripheral cholinesterase enzyme. The carbamylated enzyme eventually regenerates by natural hydrolysis and excess ACh levels revert to normal.
Clinical uses
Pyridostigmine is used to treat muscle weakness in people with myasthenia gravis and to combat the effects of curariform drug toxicity. Pyridostigmine bromide has been FDA approved for military use during combat situations as an agent to be given prior to exposure to the nerve agent Soman in order to increase survival. Used in particular during the first Gulf War, pyridostigmine bromide has been implicated as a causal factor in Gulf War syndrome.[2]
Pyridostigmine is now also used to treat orthostatic hypotension.[3]
Pyridostigmine bromide is available under the trade names Mestinon (Valeant Pharmaceuticals) and Regonol.
Contraindications
Pyrostigmine bromide is contraindicated in cases of mechanical intestinal or urinary obstruction and should be used with caution in patients with bronchial asthma.[4][5]
Side effects
Common side effects include:[4]
- Sweating
- Diarrhea
- Nausea
- Vomiting
- Abdominal cramps
- Increased salivation
- Tearing
- Increased bronchial secretions
- Constricted pupils
- Facial flushing due to vasodilation
- Erectile dysfunction
Chemistry
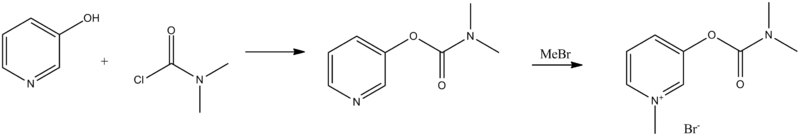
Pyridostigmine, 3-[(dimethylaminocarbonyl)oxy]-1-methyl pyridinium bromide, is synthesized from 3-hydroxypyridine, which is reacted with dimethylaminocarbamoyl chloride, which gives 3-(dimethylaminocarbamoyl)pyridine. The last is reacted with methylbromide, giving pyridostigmine.
- R. Urban, U.S. Patent 2,572,579 (1951).
References
- ^ Gulf War Syndrome: More Complex Than Middle East Politics. JWatch Psychiatry 1997;1997:15-15.
- ^ Golomb, B. (2008) "Acetylcholinesterase inhibitors and Gulf War illnesses" Proc Natl Acad Sci; Reuters; MedPageToday.com
- ^ Gales BJ, Gales MA. (2007). "Pyridostigmine in the treatment of orthostatic intolerance". Ann Pharmacother. 41 (2): 314–8. doi:10.1345/aph.1H458. PMID 17284509.
- ^ a b Mestinon | Home
- ^ Mestinon Official FDA information, side effects and uses
Related publications
- Brenner, G. M. (2000). Pharmacology. Philadelphia, PA: W.B. Saunders Company. ISBN 0-7216-7757-6
- Canadian Pharmacists Association (2000). Compendium of Pharmaceuticals and Specialties (25th ed.). Toronto, ON: Webcom. ISBN 0-919115-76-4
- Neal, M.J. (2002). Medical Pharmacology at a Glance (5th ed.). London, England: Blackwell Publishing. ISBN 1405133600
Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Acetylcholinesterase inhibitors
- Pyridines
- Carbamates
- Quaternary ammonium compounds
Wikimedia Foundation. 2010.
Look at other dictionaries:
Pyridostigmine — Structure de la pyridostigmine Général Nom IUPAC 3 [(diméthylcarbamoyl)oxy] 1 méthylpyridinium … Wikipédia en Français
pyridostigmine — noun see pyridostigmine bromide … New Collegiate Dictionary
pyridostigmine bromide — noun Etymology: International Scientific Vocabulary pyridine + neostigmine Date: 1961 a cholinergic drug C9H13BrN2O2 used especially in the treatment of myasthenia gravis and as a prophylactic against the effects of nerve gas called also… … New Collegiate Dictionary
pyridostigmine — noun A parasympathomimetic and reversible cholinesterase inhibitor, used to treat muscle weakness and to combat the effects of curariform drug toxicity … Wiktionary
pyridostigmine — pyr·i·do·stig·mine .pir əd ō stig .mēn n a cholinergic drug that is administered in the form of its bromide C9H13BrN2O2 esp. in the treatment of myasthenia gravis see MESTINON * * * n. an anticholinesterase drug used in the treatment of… … Medical dictionary
pyridostigmine — [ˌpɪrɪdə(ʊ) stɪgmi:n] noun Medicine a synthetic compound related to neostigmine, with similar but weaker and longer acting effects. Origin 1950s: blend of pyridine and neostigmine … English new terms dictionary
pyridostigmine — n. an anticholinesterase drug used in the treatment of myasthenia gravis. It is administered by mouth; side effects may include nausea, vomiting, abdominal pain, diarrhoea, sweating, and increased salivation. Trade names: Mestinon … The new mediacal dictionary
pyridostigmine — n.f. Antidote utilisé pour se protéger des effets de certains gaz de combat … Le dictionnaire des mots absents des autres dictionnaires
pyridostigmine — … Useful english dictionary
pyridostigmine bromide — /pir i doh stig meen/, Pharm. a cholinesterase inhibitor, C9H13BrN2O2, used in its bromide form in the treatment of myasthenia gravis. [1960 65; PYRID(INE) + O + (PHYSO)STIGMINE] * * * … Universalium