- O-Acetylpsilocin
-
O-Acetylpsilocin 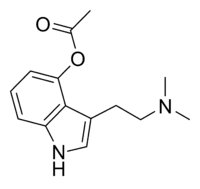
Systematic (IUPAC) name 3-[2-(Dimethylamino)ethyl]-1H-indol-4-yl acetate Clinical data Pregnancy cat. ? Legal status ? (CA) ? (US) May be considered Illegal in US if intended for human consumption (Analogue of Psilocin) Routes Oral, IV, intranasal, rectal Identifiers CAS number 92292-84-7 
ATC code None PubChem CID 15429212 ChemSpider 21106357 
Chemical data Formula C14H18N2O2 Mol. mass 246.3049 g/mol SMILES eMolecules & PubChem Physical data Melt. point 172–173 °C (342–343 °F)  (what is this?) (verify)
(what is this?) (verify)O-Acetylpsilocin, 4-acetoxy-N,N-dimethyltryptamine, psilacetin, or 4-AcO-DMT is a research chemical, psychedelic drug and has been suggested to be a potentially useful alternative to psilocybin for pharmacological studies.[1] It is the acetylated form of the psychedelic mushroom alkaloid psilocin, and is a lower homologue of 4-AcO-DET, 4-AcO-MiPT and 4-AcO-DiPT.
Contents
History
O-Acetylpsilocin (Psilacetin) is a newer psychedelic with a limited history. It is theorized to be a prodrug for psilocin, as is psilocybin, which is naturally occurring in various mushrooms. Psilacetin is O-acetylated psilocin, whereas psilocybin is O-phosphorylated.
Chemistry
O-Acetylpsilocin can be obtained by acetylation of psilocin under strongly acidic or under alkaline conditions. It is a synthetic compound. However, it is believed to be metabolized back into psilocin, which is natural and occurs in many mushrooms, most famously of the Psilocybe genus. O-Acetylpsilocin is more resistant than psilocin to oxidation under basic conditions due to its acetoxy group. While O-acetylpsilocin is fairly new and not well researched (making it a research chemical, and thus not as widely known as psilocin and psilocybin), O-acetylpsilocin is not as difficult to produce as psilocybin[citation needed], and may be of use in psychedelic research that specifically uses psilocin/psilocybin.[2]
Pharmacology
- See psilocin for more details.
In the body O-acetylpsilocin is evidently rapidly deacetylated to psilocin by deacetylases during first pass metabolism[citation needed] and during subsequent passes through the liver (evident as psilacetin is also active via parenteral routes of ingestion).
There are claims of subjective differences in effect between the acetylated and not acetylated forms of psilocin.[1] Some users report that O-acetylpsilocin lasts slightly longer than psilocin; others report that it lasts for a considerably shorter time. Many users report less body load and nausea compared to psilocin. Some users find that the visual distortions produced by O-acetylpsilocin more closely resemble those produced by DMT than those produced by psilocin. These differences could be possible if psilacetin is active itself and not merely as a prodrug. Many users, however, can not tell the difference between these two compounds when ingested.
Dosage
A dose of O-acetylpsilocin is 8 mg - 30 mg. These are slightly lower than that of the free phenol, psilocin. Some report very strong effects with 15 mg. A dose of 15 mg can produce a very profound experience, with effects such as ego loss, strong visuals (open and closed eye), and other effects similar to a high dose of psilocin. Comparison to psilocin dosage suggests that O-acetylpsilocin is somewhat more potent, and possibly more variable in terms of subjective susceptibility. O-Acetylpsilocin seems to have a more steep dose-effect curve than psilocin; therefore, caution should be taken, especially with higher doses. There is no known LD50, as there are no reported cases of fatal overdose and no known mechanism by which an excessive dose could be life-threatening.
Dosages vary slightly depending on the type of salt. The hydrochloride has a higher molecular mass and is therefore less potent on a per weight basis than its freebase form; however, freebase O-acetylpsilocin is relatively unstable and will degrade quickly at room temperature. O-Acetylpsilocin is now most commonly distributed as the fumaric acid salt (O-acetylpsilocin fumarate). The fumarate is even less potent on a per weight basis, but is also considerably more stable and will not degrade substantially at room temperature. The ratio of hydrochloride to fumarate is approximately .93:1.
O-Acetylpsilocin Fumarate Oral Dose Threshold 4 mg-7 mg Light 8 mg-12 mg Common 13 mg-17 mg Strong 18 mg-23 mg Heavy 24 mg+ These above doses are taken from the site erowid.org, which advises potential users of this chemical as a psychedelic drug: "Please note that the above doses should only be used as a general outline. This chemical is still relatively new, with a short history of human use. Every individual reacts differently to every chemical."[dubious ]
Effects
O-Acetylpsilocin is theorized to be metabolized into psilocin, as such is the case with psilocybin, however reports indicate that O-acetylpsilocin may be active on its own. The effects of O-acetylpsilocin are therefore somewhat similar to the effects of psilocybin and psilocin. Users report dose-dependent colorful visual effects and a sense of physical energy or euphoria, sometimes accompanied by abstract, associative, "trippy" thought patterns, or derealization. Other users have reported effects closer to those of an oral DMT experience.
Several available reports of O-acetylpsilocin compare it favorably to psilocybin, describing it as more euphoric, gentle, warm, and colorful. It has also been described as less jarring, and less likely to produce nausea, and it has been noted by users that O-acetylpsilocin is a somewhat sedating psychedelic. However, many of these comparisons are made with mushrooms, not pure psilocybin. In addition, it is unknown to what degree expectancy plays a role in shaping that experience.
There are several drugs that can decrease or even block the subjective effects of psychedelics, such as psilacetin/psilocybin/psilocin, 2C-x compounds, and other tryptamine and phenylethylamine based drugs that act via agonism of the 5-HT2A receptor. These include SSRI's (fluoxetine (Prozac), fluvoxamine (Luvox), paroxetine (Paxil), and drugs that block the 5-HT2A receptor such as trazodone (Desyrel), and atypical anti-psychotics including risperidone (Risperdal), olanzapine (Zyprexa), et al. Several days of abstinence from these drugs will allow clearance of 5-HT2A drugs, and up to a week may be required after stopping SSRI's to allow serotonin receptors to resensitize, and up-regulate after being suppressed by the increased serotonin levels that result from SSRI use. Of course, discontinuing these drugs may allow for the re-emergence of symtpoms of the conditions for which they were taken (if being used medically), as well as produce withdrawal/discontinuation effects, depending on the drug, dose, and duration of use.
Onset
The onset appears to last 20–40 minutes, with peak effects beginning at around 2 hours. The onset has been characterized as smoother, gentler, and more pleasant than the onset of mushrooms.
Duration
The primary effects of O-acetylpsilocin seem to typically last 4–6 hours. They have been described as lasting from 3–4 hours (low oral doses) to 8–10 hours (moderate to strong oral doses). Visual effects are described to start near the 2 hour mark, lasting up to 5 hours.
The visuals are quite mild at average doses, with mental effects dominating primarily. With closed eyes a person may perceive patterns, colors, and strange, sometimes intense subconscious awareness (perceived in a visual way). Open eyed visuals are fleeting and consist of slight movement, especially peripherally, and increased color intensity. At higher doses, full hallucinations are not uncommonly reported, but not consistent. Above 25 mg the visuals are reported to gain intensity and can include very complex geometric and dynamic patterns, "melting" of objects and loss of object comprehension (as if one was just born and seeing objects for the first time).
Legal status
4-AcO-DMT is unscheduled in the United States. It may be considered an analog of psilocin, which is a Schedule I drug under the Controlled Substances Act of the USA, and as such sale for human consumption or possession to ingest or use for illicit non-medical or industrial intents and purposes could be prosecuted as crimes under the Federal Analog Act.
O-Acetylpsilocin could also be considered illegal in the UK under the Misuse of Drugs Act 1971. Ester derivatives of prohibited substances also hold criminal penalties. O-Acetylpsilocin is an ester of psilocin, to which it metabolizes. This means that it potentially carries the same penalty as any Class A drug, such as heroin or cocaine, though the drug is still being sold over the internet in the UK as an alternative to wild mushrooms.
See also
- 4-MeO-DMT
- Psilocybin
- Tryptamine
- Indole
- Psychedelic drug
- Psychoactive drug
- Manna
- Teonanacatl
- Ayahuasca
- Soma
References
- ^ a b http://www.erowid.org/experiences/subs/exp_4AcODMT.shtml
- ^ Like psilocybin, it is metabolically converted to psilocin and is therefore thought to be nearly identical to psilocybin in effects. erowid.org[unreliable source?]
External links
- Improvements to the Synthesis of Psilocybin and a Facile Method for Preparing the O-Acetyl Prodrug of Psilocin - A publication by David E. Nichols
- Erowid 4-Acetoxy-DMT Vault
Tryptamines 1-(2-Dimethylaminoethyl)dihydropyrano(3,2-e)indole • 2-Methyl-5-HT • 4-Acetoxy-DET • 4-Acetoxy-DIPT • 4-Acetoxy-DMT • 4-HO-αMT • 4-HO-DIPT • 4-HO-MET • 4-MeO-DMT • 4-Methyl-αET • 4-Methyl-αMT • 5-Benzyloxytryptamine • 5-Bromo-DMT • 5-Carboxamidotryptamine • 5-Ethoxy-αMT • 5-Fluoro-αMT • 5-HO-αMT • 5-HTP • 5-Ethoxy-DMT • 5-Ethyl-DMT • 5-Fluoro-DMT • 5-Methyl-DMT • 5-Methoxytryptamine • 5-MeO-7,N,N-TMT • 5-Methyl-αET • 5-MeO-αET • 5-MeO-αMT • 5-MeO-DALT • 5-MeO-DET • 5-MeO-DIPT • 5-MeO-DMT • 5-MeO-DPT • 5-MeO-MALT • 5-MeO-MIPT • 5,7-Dihydroxytryptamine • 5-(Nonyloxy)tryptamine • 6-Fluoro-αMT • 7-Methyl-αET • 7-Methyl-DMT • αET • αMT • Aeruginascin • AL-37350A • BW-723C86 • Baeocystin • Bufotenidine • Bufotenin • DALT • Desformylflustrabromine • DET • DiPT • DMT • DPT • Ethocybin • EiPT • EMDT • EPT • Ethocin • FGIN-127 • FGIN-143 • Ibogaine • Indorenate • Iprocin • MET • MiPT • Miprocin • Melatonin • MS-245 • NAS • NMT • Norbaeocystin • Normelatonin • Oxypertine • PiPT • Psilocin • Psilocybin • Rizatriptan • Serotonin • Sumatriptan • Tryptamine • Tryptophan • Yohimbine • Yuremamine • Zolmitriptan
Categories:- Alkaloids
- Psychedelic tryptamines
- Entheogens
- Acetate esters
Wikimedia Foundation. 2010.
