- Dithiane
-
Dithiane 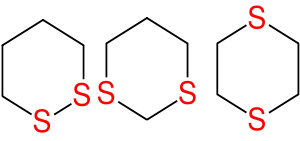


A dithiane is a heterocyclic compound composed of a cyclohexane core structure wherein two methylene units are replaced by sulfur centres. The three isomeric parent heterocycles are 1,2-dithiane, 1,3-dithiane and 1,4-dithiane.
1,3-Dithianes
1,3-Dithianes are protecting group of some carbonyl-containing compounds due to their inertness to many conditions. They form by treatment of the carbonyl compound with 1,3-propanedithiol under conditions that remove water from the system.[1] The protecting group can be removed with mercuric reagents, a process that exploits the high affinity of Hg(II) for thiolates. 1,3-Dithianes are also employed in umpolung reactions.[2] Typically, in organic synthesis, ketones and aldehydes are protected as their dioxolanes instead of dithianes.
References
- ^ E. J. Corey, D. Seebach (1988), "1,3-Dithiane", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV6P0556; Coll. Vol. 6: 556
- ^ T. W. Green, P. G. M. Wuts, "Protective Groups in Organic Synthesis" Wiley-Interscience, New York, 1999. ISBN 978-0-471-69754-1.
See also
http://www.tricity.wsu.edu/Chem542/Forposting/jo00822a019.pdf older article of historic interest for the oxidative hydrolysis of 1,3-dithianes.
http://www.organic-chemistry.org/namedreactions/corey-seebach-reaction.shtm
http://www.organic-chemistry.org/protectivegroups/carbonyl/1,3-dithiolanes.htm
This article about a heterocyclic compound is a stub. You can help Wikipedia by expanding it.
