- Phosphorus trioxide
-
Phosphorus trioxide 
 Other names
Other namesIdentifiers CAS number 1314-24-5 PubChem 123290 ChemSpider 109897 
ChEBI CHEBI:37372 
Jmol-3D images Image 1 - O1P3OP2OP(OP1O2)O3
Properties Molecular formula P4O6 Molar mass 219.88 g mol−1 Appearance colourless monoclinic crystals or liquid Density 2.135 g/cm3 Melting point 23.8 °C
Boiling point 173.1 °C
Solubility in water reacts Acidity (pKa) 9.2 Structure Molecular shape See Text Dipole moment 0 Related compounds Other anions Phosphorus trisulfide Other cations Dinitrogen trioxide
Arsenic trioxide
Antimony trioxideRelated compounds Phosphorus pentoxide
Phosphorous acid (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Phosphorus trioxide is the chemical compound with the molecular formula P4O6. Although it should properly be named tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. This colorless solid is structurally related to adamantane. It is formally the anhydride of phosphorous acid, H3PO3, but cannot be obtained by the dehydration of the acid. It is a white, waxy, crystalline and highly toxic solid[1] and has an odor similar to that of garlic.[citation needed]
Contents
Preparation
It is obtained by the combustion of phosphorus in a limited supply of air at low temperature.
- P4(s) + 3 O2(g) → P4O6(s)
By-products include red phosphorus suboxide.[1]
Chemical Properties
Phosphorus trioxide reacts with cold water to form phosphorous acid.
- P4O6(s) + 6 H2O(l) → 4 H3PO3(aq)
It reacts vigorously with hot water, via a complex set of reactions, to form red phosphorus, phosphines, H3PO3 and H3PO4.
P4O6 reacts with hydrogen chloride to form H3PO3 and phosphorus trichloride.
- P4O6 + 6 HCl → 2 H3PO3 + 2 PCl3
With chlorine or bromine it forms the corresponding phosphoryl halide, and it reacts with iodine in a sealed tube to from diphosphorus tetraiodide.[1]
As a ligand
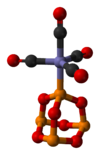
P4O6 is a ligand for transition metals, comparable to phosphite. Tetracarbonyl(tetraphosphorus hexaoxide)iron, P4O6·Fe(CO)4, is an example of a complex containing the phosphorus trioxide cage as a ligand. Its molecular structure as determined by single-crystal X-ray diffraction[2] is shown below:
References
Phosphorus compounds Categories:- Inorganic phosphorus compounds
- Sesquioxides
Wikimedia Foundation. 2010.