- Directed ortho metalation
-
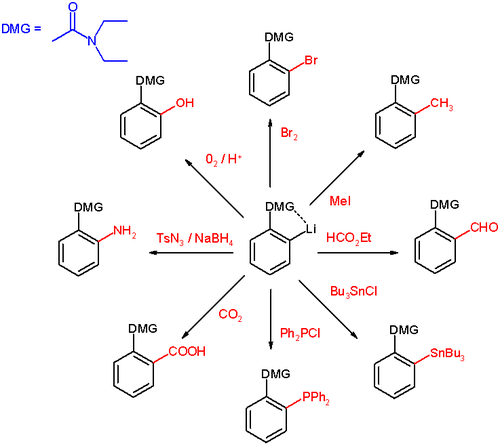
Directed ortho metalation (DoM) is an adaptation of electrophilic aromatic substitution in which electrophiles attach themselves exclusively to the ortho- position of a direct metalation group or DMG through the intermediary of an aryllithium compound [1]. The DMG interacts with lithium through a hetero atom. Examples of DMG's are the methoxy group, a tertiary amine group and an amide group.
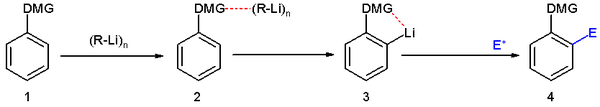
The general principle is outlined in scheme 1. An aromatic ring system with a DMG group 1 interacts with an alkyllithium such as n-butyllithium in its specific aggregation state (hence (R-Li)n) to intermediate 2 since the hetero atom on the DMG is a Lewis base and lithium the Lewis acid. The very basic alkyllithium then deprotonates the ring in the nearest ortho- position forming the aryllithium 3 all the while maintaining the acid-base interaction. An electrophile reacts in the next phase in an electrophilic aromatic substitution with a strong preference for the lithium ipso position replacing the lithium atom.
Ordinary electrophilic substitutions with an activating group show preference for both the ortho and para position, this reaction demonstrates increased regioselectivity because the ortho position alone is targeted.
This reaction type was discovered independently by Henry Gilman [2] and Georg Wittig [3] around 1940.
Scope
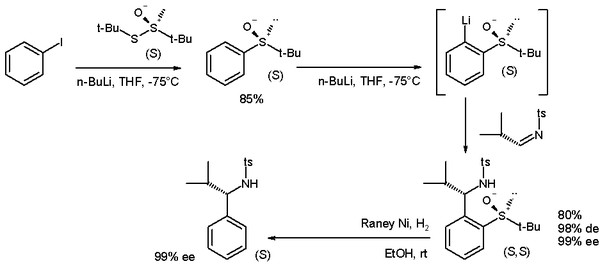
DOM has been applied to the synthesis of enantiopure benzyl amines [4] in scheme 3 [5]. On approach to the lithium intermediate, the bulky tosyl group on the imine electrophile is responsible for the asymmetric induction taking place.
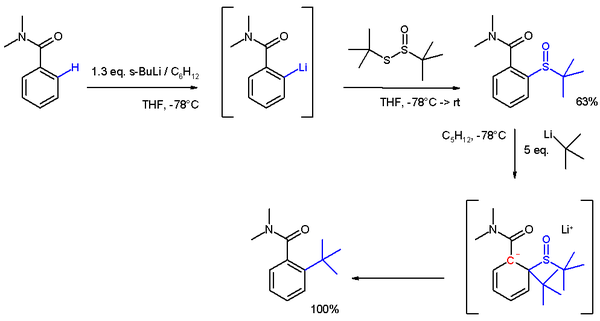
In another application [6] DOM is applied in placing a bulky tert-butyl group in an ortho position (scheme 4). The lithiation is a nucleophilic aromatic substitution and the subsequent reaction to the sulfoxide an electrophilic aromatic substitution. In the final step tert-butyllithium acts as a nucleophile in another nucleophilic aromatic substitution through an anionic intermediate.
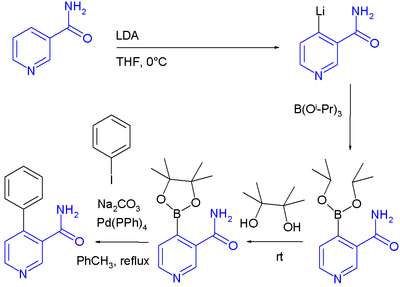
DoM has also been applied combined with a Suzuki reaction in a one-pot synthesis [7][8]:Related reaction
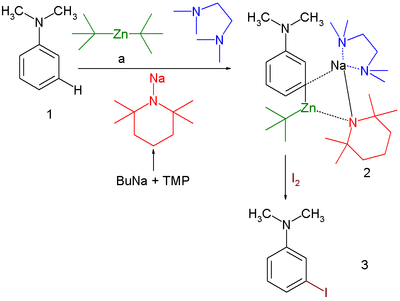
Directed metallation is not limited to lithium intermediates or even to an ortho preference. In one study [9] it is found that the reaction product of N,N-dimethylaniline with a complex of TMEDA, sodium salt of TMP and di-tert-butylzinc is a meta zincated complex as a stable crystalline compound. This complex reacts with electrophilic iodine to N,N-dimethyl-3-iodoaniline [10]:
References
- ^ Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics Victor Snieckus Chem. Rev.; 1990; 90(6); 879-933. Abstract
- ^ Relative Reactivities of Organometallic Compounds. XX.* Metalation Henry Gilman, Robert L. Bebb J. Am. Chem. Soc.; 1939; 61(1); 109-112. Abstract
- ^ G. Wittig et al. Chem. Ber. 1940, 73, 1197
- ^ ortho-Metalation of Enantiopure Aromatic Sulfoxides and Stereocontrolled Addition to Imines Nicolas Le Fur, Ljubica Mojovic, Nelly Plé, Alain Turck, Vincent Reboul, and Patrick Metzner J. Org. Chem.; 2006; 71(7) pp 2609 - 2616; Abstract
- ^ Scheme 3. Reaction scheme: reaction of iodobenzene with n-butyllithium and (S)-tert-butyl tert-butanethiosulfinate to enantiopure an sulfoxide followed by DOM reaction initiated again by n-butyllithium with electrophilic N-tosylimine. The sulfoxide group is removed by hydrogenation with Raney nickel. ts is a tosyl group, ee stands for enantiomeric excess
- ^ Contra-Friedel–Crafts tert-butylation of substituted aromatic rings via directed metallation and sulfinylation Jonathan Clayden, Christopher C. Stimson and Martine Keenan Chemical Communications, 2006, 1393 - 1394 Abstract
- ^ Directed ortho Metalation-Boronation and Suzuki-Miyaura Cross Coupling of Pyridine Derivatives: A One-Pot Protocol to Substituted Azabiaryls Manlio Alessi, Andrew L. Larkin, Kevin A. Ogilvie, Laine A. Green, Sunny Lai, Simon Lopez, and Victor Snieckus J. Org. Chem.; 2007; 72(5) pp 1588 - 1594; (Article) doi:10.1021/jo0620359
- ^ In this sequence the starting material nicotinamide is lithiated, then reacted with triisopropoxyborane to a boronate ester, then reacted with pinacol and finally reacted with iodobenzene and Tetrakis(triphenylphosphine)palladium(0)
- ^ Directed meta-Metalation Using Alkali-Metal-Mediated Zincation David R. Armstrong, William Clegg, Sophie H. Dale, Eva Hevia, Lorna M. Hogg, Gordon W. Honeyman, Robert E. Mulvey Angewandte Chemie International Edition Volume 45, Issue 23 , Pages 3775 - 3778 2006 doi:10.1002/anie.200600720
- ^ a) Solvent hexane reaction at room temperature. Selected bond lengths in 2: Zn-C bond 203.5 pm in plane with aryl plane, Na-C bond 269 pm at 76° to aryl plane
Categories:- Substitution reactions
Wikimedia Foundation. 2010.