- Aripiprazole
-
Aripiprazole 
Systematic (IUPAC) name 7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}-3,4-dihydroquinolin-2(1H)-one Clinical data Trade names Abilify AHFS/Drugs.com monograph MedlinePlus a603012 Licence data EMA:Link, US FDA:link Pregnancy cat. C (USA) Legal status ℞ Prescription only Routes oral (via tablets, orodispersable tablets, and oral solution); intramuscular Pharmacokinetic data Bioavailability 87% Protein binding >99% Metabolism liver Half-life 75h (active metabolite : 94h) Excretion feces and urine Identifiers CAS number 129722-12-9 
ATC code N05AX12 PubChem CID 60795 IUPHAR ligand 34 DrugBank DB01238 ChemSpider 54790 
UNII 82VFR53I78 
KEGG D01164 
ChEBI CHEBI:31236 
ChEMBL CHEMBL1112 
Chemical data Formula C23H27Cl2N3O2 Mol. mass 448.385 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Aripiprazole (
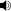 /ˌɛərɨˈpɪprəzoʊl/ air-i-pip-rə-zohl; brand names: Abilify, Aripiprex) is an atypical antipsychotic and antidepressant used in the treatment of schizophrenia, bipolar disorder, and clinical depression. It was approved by the US Food and Drug Administration (FDA) for schizophrenia on November 15, 2002; for acute manic and mixed episodes associated with bipolar disorder on October 1, 2004; as an adjunct for major depressive disorder on November 20, 2007; and to treat irritability in children with autism on 20 November 2009.[1][2] Aripiprazole was developed by Otsuka in Japan, and in the United States, Otsuka America markets it jointly with Bristol-Myers Squibb.
/ˌɛərɨˈpɪprəzoʊl/ air-i-pip-rə-zohl; brand names: Abilify, Aripiprex) is an atypical antipsychotic and antidepressant used in the treatment of schizophrenia, bipolar disorder, and clinical depression. It was approved by the US Food and Drug Administration (FDA) for schizophrenia on November 15, 2002; for acute manic and mixed episodes associated with bipolar disorder on October 1, 2004; as an adjunct for major depressive disorder on November 20, 2007; and to treat irritability in children with autism on 20 November 2009.[1][2] Aripiprazole was developed by Otsuka in Japan, and in the United States, Otsuka America markets it jointly with Bristol-Myers Squibb.Contents
Medical uses
Aripiprazole is used for the treatment of schizophrenia or bipolar disorder.[3]
Bipolar disorder
Aripiprazole has been approved by the FDA for the treatment of acute manic and mixed episodes, in both pediatric patients aged 10–17 and in adults.[4] Several double-blind, placebo-controlled trials support this use.[5][6][7][8] In addition, it is often used as maintenance therapy, either on its own or in conjunction with a mood stabilizer such as lithium or valproate. This use is also supported by a handful of studies.[9][10] Aripiprazole is at least as effective as haloperidol at reducing manic symptoms,[11][unreliable source?] and is much better tolerated by patients.[12]
Aripiprazole's use as a monotherapy in bipolar depression is more controversial. While a few pilot studies have found some effectiveness[13][14] (with one finding a reduction in anhedonia symptoms[15]), two large, double-blind, placebo-controlled studies found no difference between aripiprazole and placebo.[16] One study reported depression as a side effect of the drug.[17]
Major depression
In 2007, aripiprazole was approved by the FDA for the treatment of unipolar depression when used adjunctively with an antidepressant medication.[18] It has not been FDA-approved for use as monotherapy in unipolar depression.
Autism
In 2009, the United States FDA approved aripiprazole to treat irritability in persons with autism.[19] It was approved on the basis of two studies that showed it reduced aggression towards others, self-injury, quickly changing moods, and irritability.
Cocaine dependency
Perhaps owing to its mechanism of action relating to dopamine receptors, there is some evidence to suggest that aripiprazole blocks cocaine-seeking behaviour in animal models without significantly affecting other rewarding behaviours (such as food self-administration).[20]
Methamphetamine dependency
The book Addiction Medicine mentions studies, suggesting aripiprazole would be counter-therapeutic as treatment for methamphetamine dependency because it increased methamphetamine's stimulant and euphoric effects, and increased the baseline level of desire for methamphetamine. The author mentions studies that suggest that dopamine agonist treatment would be counter-therapeutic for methamphetamine/amphetamine dependency. [21]
Side effects
Frequent
- akathisia[22]
- headache
- agitation
- anxiety
- unusual tiredness or weakness
- nausea and vomiting
- an uncomfortable feeling in the stomach
- constipation
- increased production of saliva
- light-headedness
- insomnia
- sleepiness
- shaking
- blurred vision
- sexual dysfunction
Infrequent
- Uncontrollable twitching or jerking movements, tremors, seizure, and weight gain. Some people may feel dizzy, especially when getting up from a lying or sitting position, or may experience a fast heart rate.
- Neuroleptic malignant syndrome (Combination of fever, muscle stiffness, faster breathing, sweating, reduced consciousness, and sudden change in blood pressure and heart rate.)
- Suicidal thoughts and suicide attempts
- Painful and/or sustained erection
- Tardive dyskinesia (As with all antipsychotic medication, patients using aripiprazole may develop the permanent neurological disorder tardive dyskinesia.[23][24][25])
- Stroke (While taking aripiprazole some elderly patients with dementia have suffered from stroke or 'mini' stroke.)
- Allergic reaction (such as swelling in the mouth or throat, itching, rash), speech disorder, nervousness, agitation, fainting, reports of abnormal liver test values, inflammation of the pancreas, muscle pain, weakness, stiffness, or cramps.
Discontinuation
Aripiprazole should be discontinued gradually, with careful consideration from the prescribing doctor, to avoid withdrawal symptoms or relapse.
The British National Formulary recommends a gradual withdrawal when discontinuing anti-psychotic treatment to avoid acute withdrawal syndrome or rapid relapse.[26] Due to compensatory changes at dopamine, serotonin, adrenergic and histamine receptor sites in the central nervous system, withdrawal symptoms can occur during abrupt or over-rapid reduction in dosage. Withdrawal symptoms reported to occur after discontinuation of antipsychotics include nausea, emesis, lightheadedness, diaphoresis, dyskinesia, orthostasis, tachycardia, nervousness, dizziness, headache, excessive non-stop crying, and anxiety. The present evidence suggests that these symptoms affect a small number of susceptible individuals treated with antipsychotics.[27][28] Complicated and long-lasting rebound insomnia symptoms can also occur after withdrawing from antipsychotics.[citation needed]
Overdosage
Children or adults who ingested acute overdoses have usually manifested central nervous system depression ranging from mild sedation to coma; serum concentrations of aripiprazole and dehydroaripiprazole in these patients were elevated by up to 3-4 fold over normal therapeutic levels, yet to date no deaths have been recorded.[29]
Drug interactions
Aripiprazole is a substrate of CYP2D6 and CYP3A4. Coadministration with medications that inhibit (e.g. paroxetine, fluoxetine) or induce (e.g. carbamazepine) these metabolic enzymes are known to increase and decrease, respectively, plasma levels of aripiprazole.[30] As such, anyone taking aripiprazole should be aware that their dosage of aripiprazole may need to be decreased.
Aripiprazole may change the subjective effects of alcohol. One study[31] found that aripiprazole increased the sedative effect and reduced the sense of euphoria normally associated with alcohol consumption. However, another alcohol study[32] found that there was no difference in subjective effect between a placebo group and a group taking aripiprazole.
Pharmacology
Aripiprazole's mechanism of action is different from those of the other FDA-approved atypical antipsychotics (e.g., clozapine, olanzapine, quetiapine, ziprasidone, and risperidone). Rather than antagonizing the D2 receptor, aripiprazole acts as a D2 partial agonist (Ki = 0.34 nM).[33][34] Aripiprazole is also a partial agonist at the 5-HT1A receptor (Ki = 1.65 nM), and like the other atypical antipsychotics displays an antagonist profile at the 5-HT2A receptor (Ki = 0.8 nM).[35][36] It also antagonizes the 5-HT7 receptor (Ki = 39 nM) and acts as a partial agonist at the 5-HT2C receptor (Ki = 15 nM), both with high affinity. The latter action may underlie the minimal weight gain seen in the course of therapy.[37] Aripiprazole has moderate affinity for histamine (Ki = 61 nM), α-adrenergic (Ki = 57 nM), and D4 receptors as well as the serotonin transporter, while it has no appreciable affinity for cholinergic muscarinic receptors.[38]
D2 and D3 receptor occupancy levels are high, with average levels ranging between ~71% at 2 mg/day to ~96% at 40 mg/day.[39][40] Most atypical antipsychotics bind preferentially to extrastriatal receptors, but aripiprazole appears to be less preferential in this regard, as binding rates are high throughout the brain.[41]
Recently, it has been demonstrated that in 5-HT7 receptor knockout mice, aripiprazole does not reduce immobility time in the forced swim test (FST), and actually increases it.[42][43] This implicates 5-HT7 antagonism as playing a major role in aripiprazole's antidepressant effects, similarly to amisulpride.[42][43][44]
Aripiprazole produces 2,3-dichlorophenylpiperazine (DCPP) as a metabolite similarly to how trazodone and nefazodone reduce to 3-chlorophenylpiperazine (mCPP) and niaprazine converts to 4-fluorophenylpiperazine (pFPP).[45] It is unknown whether DCPP contributes to aripiprazole's pharmacology in any way, but the possibility cannot be excluded.
Pharmacokinetics
Aripiprazole displays linear kinetics and has an elimination half-life of approximately 75 hours. Steady-state plasma concentrations are achieved in about 14 days. Cmax (maximum plasma concentration) is achieved 3–5 hours after oral dosing. Bioavailability of the oral tablets is about 90% and the drug undergoes extensive hepatic metabolization (dehydrogenation, hydroxylation, and N-dealkylation), principally by the enzymes CYP2D6 and CYP3A4. Its only known active metabolite is dehydro-aripiprazole, which typically accumulates to approximately 40% of the aripiprazole concentration. The parenteral drug is excreted only in traces, and its metabolites, active or not, are excreted via feces and urine.[38] When dosed daily, brain concentrations of aripiprazole will increase for a period of 10–14 days, before reaching stable constant levels.[citation needed]
Patent status
Otsuka's US patent on aripiprazole expires on October 20, 2014;[46] however, due to a pediatric extension, a generic will not become available until at least April 20, 2015.[4] Barr Laboratories (now Teva Pharmaceuticals) initiated a patent challenge under the Hatch-Waxman Act in March 2007.[47] On November 15, 2010, this challenge was rejected by a United States district court in New Jersey.[1][2]
Dosage forms
 Abilify 2mg tablets (US)
Abilify 2mg tablets (US)
- Intramuscular injection, solution: 9.75 mg/mL (1.3 mL)
- Solution, oral: 1 mg/mL (150 mL) [contains propylene glycol, sucrose 400 mg/mL, and fructose 200 mg/mL; orange cream flavor]
- Tablet: 2 mg, 5 mg, 10 mg, 15 mg, 20 mg, 30 mg
- Tablet, orally disintegrating: 10 mg [contains phenylalanine 1.12 mg; creme de vanilla flavor]; 15 mg [contains phenylalanine 1.68 mg; creme de vanilla flavor]
Synthesis
References
- ^ Hitti, Miranda (20 November 2007). "FDA OKs Abilify for Depression". WebMD. http://www.webmd.com/depression/news/20071120/fda-oks-abilify-for-depression. Retrieved 8 December 2008.
- ^ Keating, Gina (23 November 2009). "FDA OKs Abilify for child autism irritability". Reuters. http://www.reuters.com/article/idUSN2023065120091121/. Retrieved 22 September 2010.
- ^ "abilify". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/abilify.html. Retrieved 3 April 2011.
- ^ a b "Patent and Exclusivity Search Results". Electronic Orange Book. US Food and Drug Administration. http://www.accessdata.fda.gov/scripts/cder/ob/docs/patexclnew.cfm?Appl_No=021436&Product_No=001&table1=OB_Rx. Retrieved 8 December 2008.
- ^ Keck PE, Marcus R, Tourkodimitris S, Ali M, Liebeskind A, Saha A, Ingenito G (September 2003). "A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania". Am J Psychiatry 160 (9): 1651–8. doi:10.1176/appi.ajp.160.9.1651. PMID 12944341. http://ajp.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=12944341.
- ^ Sachs G, Sanchez R, Marcus R, Stock E, McQuade R, Carson W, Abou-Gharbia N, Impellizzeri C, Kaplita S, Rollin L, Iwamoto T (July 2006). "Aripiprazole in the treatment of acute manic or mixed episodes in patients with bipolar I disorder: a 3-week placebo-controlled study". J. Psychopharmacol. (Oxford) 20 (4): 536–46. doi:10.1177/0269881106059693. PMID 16401666.
- ^ Vieta E, T'joen C, McQuade RD, Carson WH, Marcus RN, Sanchez R, Owen R, Nameche L (October 2008). "Efficacy of adjunctive aripiprazole to either valproate or lithium in bipolar mania patients partially nonresponsive to valproate/lithium monotherapy: a placebo-controlled study". Am J Psychiatry 165 (10): 1316–25. doi:10.1176/appi.ajp.2008.07101560. PMID 18381903.
- ^ Keck PE, Orsulak PJ, Cutler AJ, Sanchez R, Torbeyns A, Marcus RN, McQuade RD, Carson WH (January 2009). "Aripiprazole monotherapy in the treatment of acute bipolar I mania: a randomized, double-blind, placebo- and lithium-controlled study". J Affect Disord 112 (1–3): 36–49. doi:10.1016/j.jad.2008.05.014. PMID 18835043.
- ^ Keck PE, Calabrese JR, McIntyre RS, McQuade RD, Carson WH, Eudicone JM, Carlson BX, Marcus RN, Sanchez R (October 2007). "Aripiprazole monotherapy for maintenance therapy in bipolar I disorder: a 100-week, double-blind study versus placebo". J Clin Psychiatry 68 (10): 1480–91. PMID 17960961.
- ^ Keck PE, Calabrese JR, McQuade RD, Carson WH, Carlson BX, Rollin LM, Marcus RN, Sanchez R (April 2006). "A randomized, double-blind, placebo-controlled 26-week trial of aripiprazole in recently manic patients with bipolar I disorder". J Clin Psychiatry 67 (4): 626–37. doi:10.4088/JCP.v67n0414. PMID 16669728.
- ^ Young AH, Oren DA, Lowy A, McQuade RD, Marcus RN, Carson WH, Spiller NH, Torbeyns AF, Sanchez R (January 2009). "Aripiprazole monotherapy in acute mania: 12-week randomised placebo- and haloperidol-controlled study". Br J Psychiatry 194 (1): 40–8. doi:10.1192/bjp.bp.108.049965. PMID 19118324.
- ^ Vieta E, Bourin M, Sanchez R, Marcus R, Stock E, McQuade R, Carson W, Abou-Gharbia N, Swanink R, Iwamoto T (September 2005). "Effectiveness of aripiprazole v. haloperidol in acute bipolar mania: double-blind, randomised, comparative 12-week trial". Br J Psychiatry 187 (3): 235–42. doi:10.1192/bjp.187.3.235. PMID 16135860.
- ^ Mazza M, Squillacioti MR, Pecora RD, Janiri L, Bria P (December 2008). "Beneficial acute antidepressant effects of aripiprazole as an adjunctive treatment or monotherapy in bipolar patients unresponsive to mood stabilizers: results from a 16-week open-label trial". Expert Opin Pharmacother 9 (18): 3145–9. doi:10.1517/14656560802504490. PMID 19040335.
- ^ Dunn RT, Stan VA, Chriki LS, Filkowski MM, Ghaemi SN (September 2008). "A prospective, open-label study of Aripiprazole mono- and adjunctive treatment in acute bipolar depression". J Affect Disord 110 (1–2): 70–4. doi:10.1016/j.jad.2008.01.004. PMID 18272230.
- ^ Mazza M, Squillacioti MR, Pecora RD, Janiri L, Bria P (January 2009). "Effect of aripiprazole on self-reported anhedonia in bipolar depressed patients". Psychiatry Res 165 (1–2): 193–6. doi:10.1016/j.psychres.2008.05.003. PMID 18973955.
- ^ Thase ME, Jonas A, Khan A, Bowden CL, Wu X, McQuade RD, Carson WH, Marcus RN, Owen R (February 2008). "Aripiprazole monotherapy in nonpsychotic bipolar I depression: results of 2 randomized, placebo-controlled studies". J Clin Psychopharmacol 28 (1): 13–20. doi:10.1097/jcp.0b013e3181618eb4. PMID 18204335.
- ^ Muzina DJ, Momah C, Eudicone JM, Pikalov A, McQuade RD, Marcus RN, Sanchez R, Carlson BX (May 2008). "Aripiprazole monotherapy in patients with rapid-cycling bipolar I disorder: an analysis from a long-term, double-blind, placebo-controlled study". Int. J. Clin. Pract. 62 (5): 679–87. doi:10.1111/j.1742-1241.2008.01735.x. PMC 2324208. PMID 18373615. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2324208.
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021436s21,021713s16,021729s8,021866s8lbl.pdf Section 2.3 pp 7-8
- ^ Yael Waknine (24 November 2009). "FDA Approves Aripiprazole to Treat Irritability in Autistic Children". Medscape Today. WebMD. http://www.medscape.com/viewarticle/713006. Retrieved 23 February 2010.
- ^ 'Aripiprazole Blocks Reinstatement of Cocaine Seeking in an Animal Model of Relapse' Biological Psychiatry. Volume 61, Issue 5, Pages 582-590 (1 March 2007) http://www.journals.elsevierhealth.com/periodicals/bps/article/S0006-3223%2806%2900484-7/abstract
- ^ 'Addiction Medicine:Science and Practice. Author: Bankole A. Johnson. url=http://books.google.com.br/books?id=zvbr4Zn9S9MC&pg=PA145&lpg=PA145&dq=dissulfiram+stop+craving+for+amphetamines&source=bl&ots=PkBZ_o4vXK&sig=J22yh5pVYFggqKMRaiJE0Ejmmgg&hl=pt-BR&ei=7v-xToz9LsLFgAeM3r2kAQ&sa=X&oi=book_result&ct=result&resnum=2&ved=0CC0Q6AEwAQ#v=onepage&q&f=false
- ^ Basu, R; Brar, JS. "Dose-dependent rapid-onset akathisia with aripiprazole in patients with schizoaffective disorder". Neuropsychiatric disease and treatment 2 (2): 241–3. PMC 2671776. PMID 19412470. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2671776.
- ^ Abbasian C, Power P (March 2009). "A case of aripiprazole and tardive dyskinesia". J Psychopharmacol (Oxford) 23 (2): 214–5. doi:10.1177/0269881108089591. PMID 18515468.
- ^ Zaidi SH, Faruqui RA (January 2008). "Aripiprazole is associated with early onset of Tardive Dyskinesia like presentation in a patient with ABI and psychosis". Brain Inj 22 (1): 99–102. doi:10.1080/02699050701822493. PMID 18183513.
- ^ Maytal G, Ostacher M, Stern TA (June 2006). "Aripiprazole-related tardive dyskinesia". CNS Spectr 11 (6): 435–9. PMID 16816781.
- ^ Group, BMJ, ed (March 2009). "4.2.1". British National Formulary (57 ed.). United Kingdom: Royal Pharmaceutical Society of Great Britain. p. 192. ISBN 0260-535X. "Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse."
- ^ Kim, DR.; Staab, JP. (May 2005). "Quetiapine discontinuation syndrome". Am J Psychiatry 162 (5): 1020. doi:10.1176/appi.ajp.162.5.1020. PMID 15863814. http://ajp.psychiatryonline.org/cgi/content/full/162/5/1020.
- ^ Michaelides, C.; Thakore-James, M.; Durso, R. (Jun 2005). "Reversible withdrawal dyskinesia associated with quetiapine". Mov Disord 20 (6): 769–70. doi:10.1002/mds.20427. PMID 15747370.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 105-106.
- ^ "Abilify (Aripiprazole) - Warnings and Precautions". DrugLib.com. 14 February 2007. http://www.druglib.com/druginfo/abilify/warnings_precautions/. Retrieved 8 December 2008.
- ^ Kranzler, Henry R. et al. (2008). "Effects of Aripiprazole on Subjective and Physiological Responses to Alcohol". Alcoholism: Clinical and Experimental Research 32 (4): 573–579. doi:10.1111/j.1530-0277.2007.00608.x. PMC 3159685. PMID 18261195. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3159685.
- ^ Konstantin Voronin, Patrick Randall, Hugh Myrick, Raymond Anton (2008). "ARIPIPRAZOLE EFFECTS ON ALCOHOL CONSUMPTION AND SUBJECTIVE REPORTS IN A CLINICAL LABORATORY PARADIGM: POSSIBLE INFLUENCE OF SELF-CONTROL". Alcoholism: Clinical and Experimental Research 32 (11): 1954–1961. doi:10.1111/j.1530-0277.2008.00783.x. PMC 2588475. PMID 18782344. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2588475.
- ^ Lawler CP et al. (1999). "Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes". Neuropsychopharmacology 20 T. Hirose and Kikuchi, T. J. of Med. Invest. v52 suppl., Nov. 2005 (6): 612–27. doi:10.1016/S0893-133X(98)00099-2. PMID 10327430.
- ^ Burstein ES, Ma J, Wong S, Gao Y, Pham E, Knapp AE, Nash NR, Olsson R, Davis RE, Hacksell U, Weiner DM, Brann MR (December 2005). "Intrinsic Efficacy of Antipsychotics at Human D2, D3, and D4 Dopamine Receptors: Identification of the Clozapine Metabolite N-Desmethylclozapine as a D2/D3 Partial Agonist". J Pharmacol Exp Ther 315 (3): 1278–87. doi:10.1124/jpet.105.092155. PMID 16135699.
- ^ Jordan, S et al. (2002). "The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor". Eur J Pharmacol 441 (3): 137–140. doi:10.1016/S0014-2999(02)01532-7. PMID 12063084.
- ^ Shapiro, DA et al. (2003). "Aripiprazole, A Novel Atypical Antipsychotic Drug with a Unique and Robust Pharmacology". Neuropsychopharmacology 28 (8): 1400–1411. doi:10.1016/S0014-2999(02)01532-7. PMID 12063084.
- ^ Zhang JY, Kowal DM, Nawoschik SP, Lou Z, Dunlop J (February 2006). "Distinct functional profiles of aripiprazole and olanzapine at RNA edited human 5-HT2C receptor isoforms". Biochem Pharmacol 71 (4): 521–9. doi:10.1016/j.bcp.2005.11.007. PMID 16336943.
- ^ a b "Abilify (Aripiprazole) - Clinical Pharmacology". DrugLib.com. 14 February 2007. http://www.druglib.com/druginfo/abilify/pharmacology/. Retrieved 8 December 2008.
- ^ Kegeles, LS et al. (2008). "Dose–Occupancy Study of Striatal and Extrastriatal Dopamine D2 Receptors by Aripiprazole in Schizophrenia with PET and [18F]Fallypride". Neuropsychopharmacology 33 (13): 3111–3125. doi:10.1038/npp.2008.33. PMID 18418366.
- ^ Yokoi F, Gründer G, Biziere K, Stephane M, Dogan AS, Dannals RF, Ravert H, Suri A, Bramer S, Wong DF (August 2002). "Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride". Neuropsychopharmacology 27 (2): 248–59. doi:10.1016/S0893-133X(02)00304-4. PMID 12093598.
- ^ "In This Issue". Am J Psychiatry 165 (8): A46. August 2008. doi:10.1176/appi.ajp.2008.165.8.A46.
- ^ a b Hedlund PB (October 2009). "The 5-HT7 receptor and disorders of the nervous system: an overview". Psychopharmacology 206 (3): 345–54. doi:10.1007/s00213-009-1626-0. ISBN 0021300916260. PMC 2841472. PMID 19649616. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2841472.
- ^ a b Sarkisyan G, Roberts AJ, Hedlund PB (January 2010). "The 5-HT7 receptor as a mediator and modulator of antidepressant-like behavior". Behavioural Brain Research 209 (1): 99–108. doi:10.1016/j.bbr.2010.01.022. PMC 2832919. PMID 20097233. http://linkinghub.elsevier.com/retrieve/pii/S0166-4328(10)00046-X.
- ^ Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL (July 2009). "Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo". Psychopharmacology 205 (1): 119–28. doi:10.1007/s00213-009-1521-8. ISBN 0021300915218. PMC 2821721. PMID 19337725. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2821721.
- ^ Caccia S (August 2007). "N-dealkylation of arylpiperazine derivatives: disposition and metabolism of the 1-aryl-piperazines formed". Current Drug Metabolism 8 (6): 612–22. doi:10.2174/138920007781368908. PMID 17691920. http://www.bentham-direct.org/pages/content.php?CDM/2007/00000008/00000006/0005F.SGM.
- ^ Carbostyril derivatives. April 9, 1991 (published October 20, 1989)
- ^ "Barr Confirms Filing an Application with a Paragraph IV Certification for ABILIFY(R) Tablets" (Press release). Barr Pharmaceuticals, Inc.. 2007-03-20. http://phx.corporate-ir.net/phoenix.zhtml?c=60908&p=irol-newsArticle&ID=975763&highlight=. Retrieved 2008-12-23.
External links
- Abilify website
- Abilify Full Prescribing Information (for Health Care Professionals)
- U.S. National Library of Medicine: Drug Information Portal - Aripiprazole
- Abilify adverse events reported to the FDA
Antidepressants (N06A) Specific reuptake inhibitors (RIs), enhancers (REs), and releasing agents (RAs) Alaproclate • Citalopram • Escitalopram • Femoxetine • Fluoxetine# • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • Seproxetine • Sertraline# • Vilazodone • Zimelidine‡Bicifadine • Clovoxamine • Desvenlafaxine • Duloxetine • Levomilnacipran • Eclanamine • Milnacipran • Sibutramine • VenlafaxineSerotonin–norepinephrine–dopamine reuptake inhibitors (SNDRIs)Brasofensine • BTS-74,398 • Cocaine • Diclofensine • DOV-21,947 • DOV-102,677 • DOV-216,303 • EXP-561 • Fezolamine • JNJ-7925476 • NS-2359 • PRC200-SS • Pridefine • SEP-225,289 • SEP-227,162 • TesofensineAmedalin • Atomoxetine/Tomoxetine • Binedaline • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • ViloxazineDopamine reuptake inhibitors (DRIs)Amineptine • Bupropion/Amfebutamone# • Cilobamine • Manifaxine • Methylphenidate • Nomifensine • Radafaxine • TametralineNorepinephrine-dopamine releasing agents (NDRAs)Serotonin-norepinephrine-dopamine releasing agents (SNDRAs)4-Methyl-αMT • αET/Etryptamine • αMT/MetryptamineOthersIndeloxazine • Teniloxazine • Tramadol • ViqualineReceptor antagonists and/or reuptake inhibitors Serotonin antagonists and reuptake inhibitors (SARIs)Serotonin modulators and stimulators (SMSs)VortioxetineTricyclic and tetracyclic antidepressants (TCAs/TeCAs) TricyclicsAmezepine • Amineptine • Amitriptyline# • Amitriptylinoxide • Azepindole • Butriptyline • Cianopramine • Clomipramine • Cotriptyline • Cyanodothiepin • Demexiptiline • Depramine/Balipramine • Desipramine • Dibenzepin • Dimetacrine • Dosulepin/Dothiepin • Doxepin • Enprazepine • Fluotracen • Hepzidine • Homopipramol • Imipramine • Imipraminoxide • Intriptyline • Iprindole • Ketipramine • Litracen • Lofepramine • Losindole • Mariptiline • Melitracen • Metapramine • Mezepine • Naranol • Nitroxazepine • Nortriptyline • Noxiptiline • Octriptyline • Opipramol • Pipofezine • Propizepine • Protriptyline • Quinupramine • Tampramine • Tianeptine • Tienopramine • Trimipramine;7-OH-Amoxapine • Amoxapine • Aptazapine • Azipramine • Ciclazindol • Ciclopramine • Esmirtazapine • Loxapine • Maprotiline • Mazindol • Mianserin • Mirtazapine • Oxaprotiline • Setiptiline/TeciptilineMonoamine oxidase inhibitors (MAOIs) NonselectiveIrreversible: Benmoxin • Echinopsidine • Iproclozide • Iproniazid • Isocarboxazid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Safrazine • Tranylcypromine; Reversible: Caroxazone • Paraxazone;• Quercetin;MAOA-SelectiveIrreversible: Clorgiline; Reversible: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Esuperone • Harmala Alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima;MAOB-SelectiveIrreversible: Ladostigil • Mofegiline • Pargyline • Rasagiline • Selegiline; Reversible: Lazabemide • MilacemideAzapirones and other 5-HT1A receptor agonists Alnespirone • Aripiprazole • Befiradol • Buspirone • Eptapirone • Flesinoxan • Flibanserin • Gepirone • Ipsapirone • Oxaflozane • Tandospirone • Vilazodone • ZalospironeAdrenergics Receptor ligands Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Cannabivarin • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.βAgonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • XipranololReuptake inhibitors Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • melitracen • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Ginkgo biloba • Indeloxazine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Tedatioxetine • Teniloxazine • Tramadol • ZiprasidoneReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-OH-PEA • 4-CAB • 4-FA • 4-FMA • 4-MA • 4-MMA • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Me-PEA • BDB • Benzphetamine • BOH • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • DMA • DMMA • EBDB • Ephedrine • Ethcathinone • Ethylamphetamine • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • IAP • IMP • L-Deprenyl (Selegiline) • Lisdexamfetamine • Lophophine • MBDB • MDA (Tenamfetamine) • MDEA • MDMA • MDMPEA • MDOH • MDPEA • Mefenorex • Mephedrone • Mephentermine • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methcathinone • Methedrone • Methylone • NAP • Ortetamine • Paredrine • pBA • pCA • Pentorex (Phenpentermine) • Phenethylamine • Pholedrine • Phenpromethamine • Phentermine • Phenylpropanolamine • pIA • Prenylamine • Propylamphetamine • Pseudoephedrine • Tiflorex • Tyramine • Xylopropamine • Zylofuramine; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • pFPP; Others: 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-Benzylpiperidine • 4-Benzylpiperidine • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanorex • Isometheptene • Methylhexanamine • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • TuaminoheptaneEnzyme inhibitors 3,4-DihydroxystyreneDBHCGS-19281A • SKF-64139 • SKF-7698Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline • Safinamide
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.COMTOthers Ferrous Iron (Fe2+) • S-Adenosyl-L-Methionine • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal Phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)List of adrenergic drugsDopaminergics Receptor ligands AgonistsAdamantanes: Amantadine • Memantine • Rimantadine; Aminotetralins: 7-OH-DPAT • 8-OH-PBZI • Rotigotine • UH-232; Benzazepines: 6-Br-APB • Fenoldopam • SKF-38,393 • SKF-77,434 • SKF-81,297 • SKF-82,958 • SKF-83,959; Ergolines: Bromocriptine • Cabergoline • Dihydroergocryptine • Lisuride • LSD • Pergolide; Dihydrexidine derivatives: 2-OH-NPA • A-86,929 • Ciladopa • Dihydrexidine • Dinapsoline • Dinoxyline • Doxanthrine; Others: A-68,930 • A-77,636 • A-412,997 • ABT-670 • ABT-724 • Aplindore • Apomorphine • Aripiprazole • Bifeprunox • BP-897 • CY-208,243 • Dizocilpine • Etilevodopa • Flibanserin • Ketamine • Melevodopa • Modafinil • Pardoprunox • Phencyclidine • PD-128,907 • PD-168,077 • PF-219,061 • Piribedil • Pramipexole • Propylnorapomorphine • Pukateine • Quinagolide • Quinelorane • Quinpirole • RDS-127 • Ro10-5824 • Ropinirole • Rotigotine • Roxindole • Salvinorin A • SKF-89,145 • Sumanirole • Terguride • Umespirone • WAY-100,635AntagonistsTypical antipsychotics: Acepromazine • Azaperone • Benperidol • Bromperidol • Clopenthixol • Chlorpromazine • Chlorprothixene • Droperidol • Flupentixol • Fluphenazine • Fluspirilene • Haloperidol • Loxapine • Mesoridazine • Methotrimeprazine • Nemonapride • Penfluridol • Perazine • Periciazine • Perphenazine • Pimozide • Prochlorperazine • Promazine • Sulforidazine • Sulpiride • Sultopride • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Trifluperidol • Zuclopenthixol; Atypical antipsychotics: Amisulpride • Asenapine • Blonanserin • Cariprazine • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Lurasidone • Melperone • Molindone • Mosapramine • Olanzapine • Paliperidone • Perospirone • Piquindone • Quetiapine • Remoxipride • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Antiemetics: AS-8112 • Alizapride • Bromopride • Clebopride • Domperidone • Metoclopramide • Thiethylperazine; Others: Amoxapine • Buspirone • Butaclamol • Ecopipam • EEDQ • Eticlopride • Fananserin • L-745,870 • Nafadotride • Nuciferine • PNU-99,194 • Raclopride • Sarizotan • SB-277,011-A • SCH-23,390 • SKF-83,959 • Sonepiprazole • Spiperone • Spiroxatrine • Stepholidine • Tetrahydropalmatine • Tiapride • UH-232 • YohimbineReuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • Tedatioxetine • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugsHistaminergics Receptor
ligandsAgonists: 2-Pyridylethylamine • Betahistine • Histamine • HTMT • UR-AK49
Antagonists: 1st generation: 4-Methyldiphenhydramine • Alimemazine • Antazoline • Azatadine • Bamipine • Benzatropine (Benztropine) • Bepotastine • Bromazine • Brompheniramine • Buclizine • Captodiame • Carbinoxamine • Chlorcyclizine • Chloropyramine • Chlorothen • Chlorphenamine • Chlorphenoxamine • Cinnarizine • Clemastine • Clobenzepam • Clocinizine • Cyclizine • Cyproheptadine • Dacemazine • Deptropine • Dexbrompheniramine • Dexchlorpheniramine • Dimenhydrinate • Dimetindene • Diphenhydramine • Diphenylpyraline • Doxylamine • Embramine • Etybenzatropine (Ethylbenztropine) • Etymemazine • Histapyrrodine • Homochlorcyclizine • Hydroxyethylpromethazine • Hydroxyzine • Isopromethazine • Isothipendyl • Meclozine • Mepyramine (Pyrilamine) • Mequitazine • Methafurylene • Methapyrilene • Methdilazine • Moxastine • Niaprazine • Orphenadrine • Oxatomide • Oxomemazine • Phenindamine • Pheniramine • Phenyltoloxamine • Pimethixene • Piperoxan • Promethazine • Propiomazine • Pyrrobutamine • Talastine • Thenalidine • Thenyldiamine • Thiazinamium • Thonzylamine • Tolpropamine • Tripelennamine • Triprolidine; 2nd generation: Acrivastine • Astemizole • Azelastine • Cetirizine • Clemizole • Clobenztropine • Ebastine • Emedastine • Epinastine • Ketotifen • Latrepirdine • Levocabastine • Loratadine • Mebhydrolin • Mizolastine • Olopatadine • Rupatadine • Setastine • Terfenadine; "3rd generation": Desloratadine • Fexofenadine • Levocetirizine; Miscellaneous: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc) • Tetracyclic antidepressants (Mianserin, Mirtazapine, etc) • Serotonin antagonist and reuptake inhibitors (Trazodone, Nefazodone) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc) • Atypical antipsychotics (Clozapine, Olanzapine, Quetiapine, etc)Agonists: Amthamine • Betazole • Dimaprit • Histamine • HTMT • Impromidine • UR-AK49
Antagonists: Burimamide • Cimetidine • Ebrotidine • Famotidine • Lafutidine • Lavoltidine/Loxtidine • Lupitidine • Metiamide • Niperotidine • Nizatidine • Oxmetidine • Ranitidine • RoxatidineAgonists: α-Methylhistamine • Cipralisant • Histamine • Imetit • Immepip • Immethridine • Methimepip • Proxyfan
Antagonists: A-349,821 • A-423,579 • ABT-239 • Betahistine • Burimamide • Ciproxifan • Clobenpropit • Conessine • GSK-189,254 • Impentamine • Iodophenpropit • JNJ-5,207,852 • MK-0249 • NNC-38-1,049 • PF-03654746 • Pitolisant • SCH-79,687 • Thioperamide • VUF-5,681Agonists: 4-Methylhistamine • Histamine • VUF-8,430
Antagonists: JNJ-7,777,120 • Thioperamide • VUF-6,002Reuptake
inhibitorsVMAT inhibitorsEnzyme
inhibitorsHDC inhibitorsCatechin • Meciadanol • Naringenin • TritoqualineHNMT inhibitorsDAO inhibitorsAminoguanidineOthers L-HistidineSerotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969 • Vortioxetine
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507 • VortioxetineReuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DCA • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersPiperazines Simple piperazines
(no additional rings)1-Cyclohexylpiperazine • Aminoethylpiperazine • Diethylcarbamazine • HEPPS • Midafotel • Piperazine • PIPESPhenylpiperazines Acaprazine • Antrafenine • Aripiprazole • Batoprazine • Bifeprunox • BRL-15,572 • Ciprofloxacin • CSP-2503 • Dapiprazole • DCPP • DMPP • Diphenylpiperazine • Dropropizine • EGIS-12,233 • Elopiprazole • Eltoprazine • Enpiprazole • Ensaculin • Etoperidone • Flesinoxan • Flibanserin • Fluprazine • Itraconazole • Ketoconazole • Levodropropizine • Lorpiprazole • mCPP • Mefway • MeOPP • Mepiprazole • Naftopidil • Naphthylpiperazine • Nefazodone • Niaprazine • Oxypertine • Pardoprunox • pCPP • pFPP • Posaconazole • PRX-00023 • S-14,506 • S-14,671 • S-15,535 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • Sonepiprazole • TFMPP • Tolpiprazole • Trazodone • Urapidil • Vesnarinone • Vilazodone • WAY-100,135 • WAY-100,635Benzylpiperazines 2C-B-BZP • Befuraline • Bifeprunox • Buclizine • BZP • Chlorbenzoxamine • DBZP • Fipexide • Imatinib • MBZP • MDBZP • Meclozine • Piberaline • Piribedil • Trimetazidine • VesnarinoneDiphenylalkylpiperazines
(benzhydrylalkylpiperazines)Almitrine • Amperozide • BRL-15,572 • Buclizine • BW373U86 • Cetirizine • Chlorbenzoxamine • Chlorcyclizine • Cinnarizine • Clocinizine • Cyclizine • DBL-583 • Diphenylmethylpiperazine • Dotarizine • DPI-221 • DPI-287 • DPI-3290 • GBR-12,783 • GBR-12,935 • GBR-13,069 • GBR-13,098 • GBR-13,119 • Hydroxyzine • Lidoflazine • Manidipine • Meclozine • Oxatomide • SNC-80 • VanoxerinePyrimidinylpiperazines Buspirone • Dasatinib • Eptapirone • Gepirone • Ipsapirone • Piribedil • Pyrimidinylpiperazine • Revospirone • Tandospirone • Tirilazad • Trimazosin • Umespirone • ZalospironePyridinylpiperazines Atevirdine • Azaperone • PyridinylpiperazineBenzo(iso)thiazolylpiperazines Tricyclics
(piperazine attached via side chain)Others 6-Nitroquipazine • Azimilide • Cinepazet • Cyclohexylpiperazine • Hexocyclium • Indinavir • JNJ-7777120 • Lodenafil • Mirodenafil • PB-28 • Quipazine • Ranolazine • SA-4503 • Sildenafil • Tadalafil • Vardenafil • VUF-6002 • ZipeprolCategories:- Neurophysiology
- Signal transduction
- Atypical antipsychotics
- Piperazines
- Bristol-Myers Squibb
- Organochlorides
- Phenol ethers
- Quinolones
- Lactams
Wikimedia Foundation. 2010.