- Procaine
-
"Novocaine" redirects here. For other uses, see Novocaine (disambiguation).
Procaine 

Systematic (IUPAC) name 2-(diethylamino)ethyl 4-aminobenzoate Clinical data AHFS/Drugs.com monograph Pregnancy cat. B2(AU) C(US) Legal status Prescription Only (S4) (AU) Routes Parenteral Pharmacokinetic data Bioavailability n/a Metabolism Hydrolysis by plasma esterases Half-life 40–84 seconds Excretion Renal Identifiers CAS number 59-46-1 
ATC code N01BA02 C05AD05 S01HA05 PubChem CID 4914 DrugBank DB00721 ChemSpider 4745 
UNII 4Z8Y51M438 
KEGG D08422 
ChEBI CHEBI:8430 
ChEMBL CHEMBL569 
Chemical data Formula C13H20N2O2 Mol. mass 236.31 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Procaine is a local anesthetic drug of the amino ester group. It is used primarily to reduce the pain of intramuscular injection of penicillin, and it was also used in dentistry. Owing to the ubiquity of the trade name Novocain, in some regions procaine is referred to generically as novocaine. It acts mainly by being a sodium channel blocker.[1]
Procaine was first synthesized in 1905,[2] shortly after amylocaine, and is the oldest man-made local anesthetic still in clinical use for injection.[3] It was created by the German chemist Alfred Einhorn who gave the chemical the trade name Novocaine, from the Latin nov- (meaning new) and -caine, a common ending for alkaloids used as anesthetics. It was introduced into medical use by surgeon Heinrich Braun. Prior to the discovery of Stovaine and Novocaine, cocaine was the most commonly used local anesthetic.
Procaine is used less frequently today since more effective (and hypoallergenic) alternatives such as lidocaine (Xylocaine) exist. Like other local anesthetics (such as mepivacaine, and prilocaine), procaine is a vasodilator, and is often coadministered with epinephrine for the purpose of vasoconstriction. Vasoconstriction helps to reduce bleeding and prevents the drug from reaching systemic circulation in large amounts. Also unlike cocaine, procaine does not have the euphoric and addictive qualities that put it at risk for abuse. Cocaine is also not a vasodilator.
Procaine, an ester anesthetic, is metabolized in the plasma by the enzyme pseudocholinesterase through hydrolysis into para-amino benzoic acid (PABA), which is then excreted by the kidneys into the urine. Allergic reactions to procaine are usually not in response to procaine itself, but to PABA. About 1 in 3000 people have an atypical form of pseudocholinesterase, which does not hydrolyze ester anesthetics such as procaine, resulting in a prolonged period of high levels of the anesthetic in the blood and increased toxicity.
Procaine is the primary ingredient in the controversial preparation Gerovital H3 by Ana Aslan (Romania), which is claimed by its advocates to remedy many effects of aging. The mainstream medical view is that these claims were seriously studied and discredited in the 1960s.
1% Procaine injection has been recommended for the treatment of extravasation complications associated with venipuncture (along with moist heat, ASA, steroids, antibiotics). It has likewise been recommended for treatment of inadvertent intra-arterial injections (10mL of 1% procaine) as it helps relieve pain and vascular spasm.
Procaine is occasionally added as an additive in illicit street drugs such as cocaine. It is also used by professional dominatrices to enhance BDSM play.[4]
Contents
Adverse effects
Application of procaine leads to the depression of neuronal activity. The depression causes the nervous system to become hypersensitive producing restlessness and shaking leading to minor to severe convulsions. Studies on animals have shown that the use of procaine led to the increase of dopamine and serotonin levels in the brain.[5] Procaine can also cause allergic reactions causing the individuals to have problems with breathing, rashes, and swelling. Other issues may occur because of varying individual tolerance to procaine dosage. Nervousness and dizziness can arise from the excitation central nervous system which may lead to respiratory failure if overdosed. Procaine may also induce weakening of the myocardium leading to cardiac arrest.[6].
Chemistry
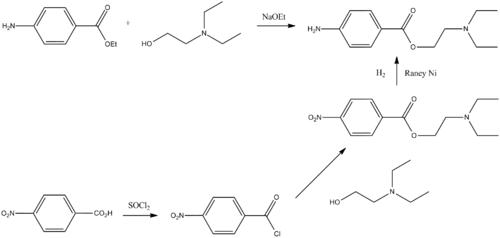
Procaine, the 2-diethylaminoethyl ester of 4-aminobenzoic acid, is synthesized in two ways. The first way consists of the direct reaction of the 4-aminobenzoic acid ethyl ester with 2-diethylaminoethanol in the presence of sodium ethoxide. The second way is by reacting 4-nitrobenzoic acid with thionyl chloride, the resulting acid chloride is then esterified with 2-diethylaminoethanol. Finally, the nitro group is reduced by hydrogenation over Raney nickel catalyst.
See also
References
- ^ DrugBank - Showing drug card for Procaine (DB00721) Update Date 2009-06-23
- ^ Ritchie, J. Murdoch; Greene, Nicholas M. (1990). "Local Anesthetics". In Gilman, Alfred Goodman; Rall, Theodore W.; Nies, Alan S. et al.. Goodman and Gilman's The Pharmacological Basis of Therapeutics (8 ed.). New York: Pergamon Press. p. 311. ISBN 0080402968.
- ^ R. Minard, "The Preparation of the Local Anesthetic, Benzocaine, by an Esterification Reaction", Adapted from Introduction to Organic Laboratory Techniques: A Microscale Approach, Pavia, Lampman, Kriz & Engel, 1989. Revised 10/18/06
- ^ http://www.klinikerotik.de/en/internal-message.html
- ^ Sawaki, K., and Kawaguchim, M. "Some Correlations between procaine-induced convulsions and monoamides in the spinal cord of rats". Japanese Journal of Pharmacology,51(3), 1989, p. 369-376.
- ^ http://www.drugs.com/pro/novocain.html - Novocain Official FDA information. Updated(08/2007)
Further reading
- Hahn-Godeffroy, J.D.: Wirkungen und Nebenwirkungen von Procain: was ist gesichert?. Komplement. integr. Med. 02/2007, 32-34.
- A. Einhorn, K. Fiedler, C. Ladish, E. Uhlfelder. Justus Liebigs Annalen der Chemie 371, 125, 131, 142, 162 (1910).
- A. Einhorn, M. Lucius, U.S. Patent 812,554 (1906).
- M. Lucius, DE 179627 (1904).
- M. Lucius, DE 194748 (1905).
Vasoprotectives (C05) Antihemorrhoidals for topical use corticosteroids (Hydrocortisone, Prednisolone, Betamethasone, Fluorometholone, Fluocortolone, Dexamethasone, Fluocinolone acetonide, Fluocinonide)
local anesthetics (Lidocaine, Tetracaine, Benzocaine, Cinchocaine, Procaine, Oxetacaine, Pramocaine)
other (Tribenoside)Antivaricose therapy heparins or heparinoids for topical use (Organo-heparinoid, Sodium apolate, Heparin, Pentosan polysulfate)
sclerosing agents for local injection (Monoethanolamine oleate, Polidocanol, Invert sugar, Sodium tetradecyl sulfate, Phenol)
other (Calcium dobesilate)Capillary stabilising agents bioflavonoids (Rutoside, Monoxerutin, Diosmin, Troxerutin, Hidrosmin) - other (Tribenoside, Etamsylate)Anesthetics: Local anesthetics - primarily sodium channel blockers (N01B) Esters Esters of aminobenzoic acidAmylocaine • Benzocaine • Butacaine • Butamben • Chloroprocaine • Dimethocaine • Meprylcaine • Metabutoxycaine • Orthocaine • Propoxycaine • Procaine (Novocaine) • Proxymetacaine • Risocaine • TetracaineEsters of benzoic acidAmides Articaine • Bupivacaine # /Levobupivacaine/Ropivacaine • Carticaine • Cinchocaine • Etidocaine • Lidocaine # • Mepivacaine • Prilocaine • TrimecaineCombinations Categories:- Dental equipment
- Local anesthetics
- 1905 introductions
- Benzoates
- Anilines
Wikimedia Foundation. 2010.