- Curtin–Hammett principle
-
The Curtin–Hammett principle is a principle in chemical kinetics proposed by David Yarrow Curtin and Louis Plack Hammett. It states that, for a reaction that has a pair of reactive intermediates or reactants that interconvert rapidly (as is usually the case for conformers), each going irreversibly to a different product, the product ratio will depend only on the difference in the free energy of the transition state going to each product, and will not necessarily reflect the equilibrium distribution of the two intermediates.[1][2]
Contents
Definition
The Curtin–Hammett principle applies to systems in which different products are formed from two substrates in equilibrium with one another. The rapidly interconverting reactants can be enantiomers, diastereomers, or constitutional isomers. Product formation must be irreversible, and the different products must be unable to interconvert.[3]
For example, given species A and B that equilibrate rapidly while A turns irreversibly into C, and B turns irreversibly into D:
K is the equilibrium constant between A and B, and k1 and k2 are the rate constants for the formation of C and D, respectively. When the rate of interconversion between A and B is much faster than either k1 or k2, then the Curtin–Hammett principle tells us that the C:D product ratio is not equal to the A:B reactant ratio, but is instead determined by the relative energy of the transition states.
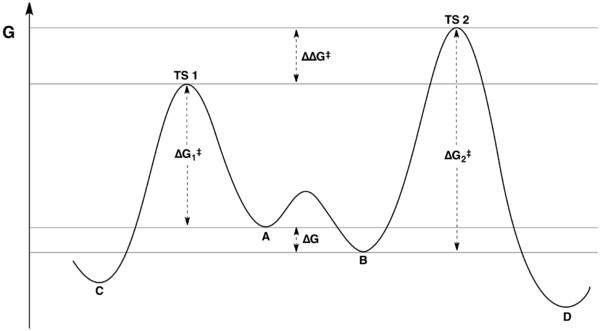
The reaction coordinate free energy profile can be represented by the following scheme:
The ratio of products depends on the value labeled ΔΔG‡ in the figure: C will be the major product, because the energy of TS1 is lower than the energy of TS2. The commonly made assertion that the product distribution does not in any way reflect the relative free energies of substrates A and B is incorrect, however.[3][4] As shown in the derivation below, the product ratio can be expressed solely as a function of ΔΔG‡, or as a function of K, k1, and k2.
Derivation
A generic reaction under Curtin-Hammett can be described by the following parameters:
The rate of formation for compound C from A is given as
and that of D from B as:
with Kc the equilibrium constant. The ratio of the rates is then:
Because equilibration is fast compared to product formation, the ratio [B]/[A] remains unchanged throughout the reaction and K is constant. The product ratio can therefore be written as:
Classes of reactions under Curtin-Hammett control
Three main classes of reactions can be explained by the Curtin–Hammett principle: either the more or less stable conformer may react more quickly, or they may both react at the same rate.
Case I: More stable conformer reacts more quickly
One category of reactions under Curtin-Hammett control includes transformations in which the more stable conformer reacts more quickly. This occurs when the transition state from the major intermediate to its respective product is lower in energy than the transition state from the minor intermediate to the other possible product. The major product is then derived from the major conformer, and the product distribution does not mirror the equilibrium conformer distribution.
Example: Piperidine Oxidation
An example of a Curtin-Hammett scenario in which the more stable conformational isomer reacts more quickly is observed during the oxidation of piperidines. In the case of N-methyl piperidine, inversion at nitrogen between diasteriomeric conformers is much faster than the rate of amine oxidation.[5] The conformation which places the methyl group in the equatorial position is 3.16 kcal/mol more stable than the equatorial conformation.[6] The product ratio of 95:5 indicates that the more stable conformer leads to the major product.[7]
Case II: Less stable conformer reacts more quickly
A second category of reactions under Curtin-Hammett control includes those in which the less stable conformer reacts more quickly. In this case, despite an energetic preference for the less reactive species, the major product is derived from the higher-energy species. An important implication is that the product of a reaction can be derived from a conformer that is at sufficiently low concentration as to be unobservable in the ground state.[3]
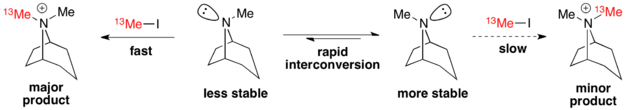
Example: Tropane Alkylation
The alkylation of tropanes with methyl iodide is a classic example of a Curtin-Hammett scenario in which a major product can arise from a less stable conformation.[3] Here, the less stable conformer reacts via a more stable transition state to form the major product.[8] Therefore, the ground state conformational distribution does not reflect the product distribution.
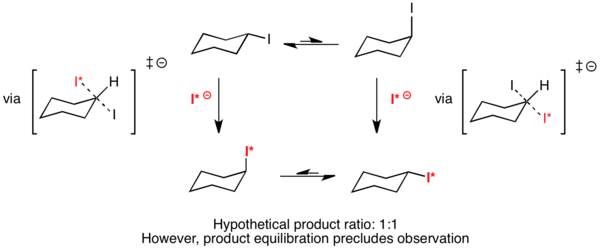
Case III: Both conformers react at the same rate
It is hypothetically possible that two different conformers in equilibrium could react through transition states that are equal in energy. In this case, both conformers would react at the same rate. Eliel has proposed that the hypothetical reaction of cyclohexyl iodide with radiolabeled iodide would result in a completely symmetric transition state.[9] Because both the equatorial and axial-substituted conformers would react through the same transition state, ΔΔG‡ would equal zero. By the Curtin–Hammett principle, the distribution of products should then be 50% axial substituted and 50% equatorial substituted. However, equilibration of the products precludes observation of this phenomenon.[3]
Application to stereoselective reactions
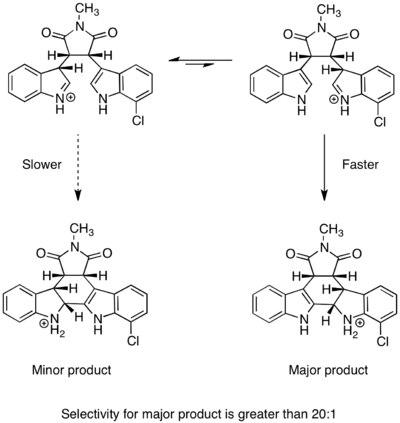
The Curtin–Hammett principle is used to explain the selectivity ratios for some stereoselective reactions.
Application to dynamic kinetic resolution
The Curtin–Hammett principle can explain the observed dynamics in transformations employing dynamic kinetic resolution, such as the Noyori asymmetric hydrogenation[10] and enantioselective lithiation.[11]
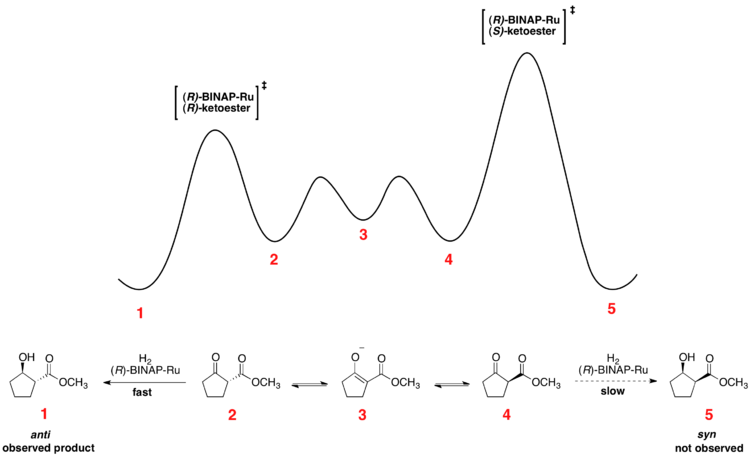
Noyori asymmetric hydrogenation
Rapid equilibration between enantiomeric conformers and irreversible hydrogenation place the reaction under Curtin-Hammett control. The use of a chiral catalyst results in a higher-energy and a lower-energy transition state for hydrogenation of the two enantiomers. The transformation occurs via the lower-energy transition state to form the product as a single enantiomer.[12] Consistent with the Curtin–Hammett principle, the ratio of products depends on the absolute energetic barrier of the irreversible step of the reaction, and does not reflect the equilibrium distribution of substrate conformers. The relative free energy profile of one example of the Noyori asymmetric hydrogenation is shown below:
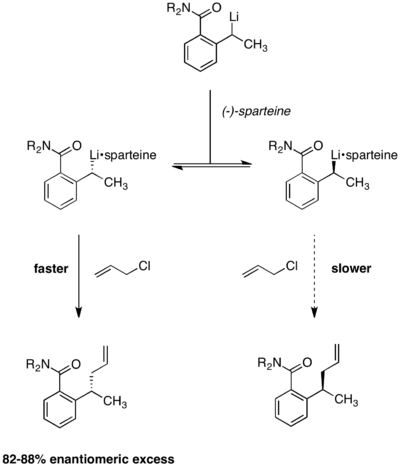
Enantioselective lithiation
Dynamic kinetic resolution under Curtin-Hammett conditions has also been applied to enantioselective lithiation reactions. In the reaction below, it was observed that product enantioselectivities were independent of the chirality of the starting material. The use of (-)-sparteine is essential to enantioselectivity, with racemic product being formed in its absence.[11] Equilibration between the two alkyllithium complexes was demonstrated by the observation that enantioselectivity remained constant over the course of the reaction. Were the two reactant complexes not rapidly interconverting, enantioselectivity would erode over time as the faster-reacting conformer was depleted.
Synthetic applications
The Curtin–Hammett principle has been invoked to explain selectivity in a variety of synthetic pathways. One example is observed en route to the antitumor antibiotic AT2433-A1, in which a Mannich-type cyclization proceeds with excellent regioselectivity. Studies demonstrate that the cyclization step is irreversible in the solvent used to run the reaction, suggesting that Curtin-Hammett kinetics can explain the product selectivity.[13]
References
- ^ Carey, Francis A.; Sundberg, Richard J.; (1984). Advanced Organic Chemistry Part A Structure and Mechanisms (2nd ed.). New York N.Y.: Plenum Press. ISBN 0-306-41198-9
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (1994) "Curtin–Hammett principle".
- ^ a b c d e Jeffrey I. Seeman (1983). "Effect of Conformational Change on Reactivity in Organic Chemistry. Evaluations, Application, and Extensions of Cutin-Hammett/Winstein-Holness Kinetics". Chemical Reviews 83 (2): 83–134. doi:10.1021/cr00054a001.
- ^ Jeffrey I. Seeman (1986). "The Curtin-Hammett Principle and the Winstein-Holness Equation". Journal of Chemical Education 63 (1): 42–48. doi:10.1021/ed063p42.
- ^ P. J. Crowley, M. J. T. Robinson and M. G. Ward (1977). "Conformational effects in compounds with 6-membered rings-XII". Tetrahedron 33 (9): 915–925. doi:10.1016/0040-4020(77)80202-0.
- ^ Luis Carballeira, Ignacio Pérez-Juste (1998). "Influence of calculation level and effect of methylation on axial/equatorial equilibria in piperidines". Journal of Computational Chemistry 19 (8): 961–976. doi:10.1002/(SICI)1096-987X(199806)19:8<961::AID-JCC14>3.0.CO;2-A.
- ^ Y. Shvo and E.D. Kaufman (1972). "Configurational and conformational analysis of cyclic amine oxides". Tetrahedron 28 (3): 573–580. doi:10.1016/0040-4020(72)84021-3.
- ^ Rodney D. Otzenberger, Kenneth B. Lipkowitz, Bradford P. Mundy (1974). "Quaternizations in the 8-azabicyclo[4.3.0]non-3-ene series". Journal of Organic Chemistry 39 (3): 319–321. doi:10.1021/jo00917a008.
- ^ Eliel, E. L. “Stereochemistry of Carbon Compounds”; McGraw-Hill: New York, 1962, pp 149-156, 234-239.
- ^ M. Kitamura, M. Tokunaga, R. Noyori (1993). "Quantitative expression of dynamic kinetic resolution of chirally labile enantiomers: stereoselective hydrogenation of 2-substituted 3-oxo carboxylic esters catalyzed by BINAP-ruthenium(II) complexes". Journal of the American Chemical Society 115 (1): 144–152. doi:10.1021/ja00054a020.
- ^ a b Peter Beak, Amit Basu, Donald J. Gallagher, Yong Sun Park, and S. Thayumanavan (1996). "Regioselective, Diastereoselective, and Enantioselective Lithiation−Substitution Sequences: Reaction Pathways and Synthetic Applications". Accounts of Chemical Research 29 (11): 552–560. doi:10.1021/ar950142b.
- ^ Noyori, Ryōji; Ikeda, T.; Ohkuma, T.; Widhalm, M.; Kitamura, M.; Takaya, H.; Akutagawa, S.; Sayo, N.; Saito, T.; Taketomi, T.; Kumobayashis, H. (1989). "Stereoselective hydrogenation via dynamic kinetic resolution". Journal of the American Chemical Society 111 (25): 9134–9135. doi:10.1021/ja00207a038.
- ^ Chisholm, J. M.; Van Vanken, D. L. (2000). "Regiocontrolled synthesis of the antitumor antibiotic AT2433-A1". Journal of Organic Chemistry 65 (22): 7541–7553. doi:10.1021/jo000911r.
External links
See also
Categories:
Wikimedia Foundation. 2010.


![\frac{d[C]}{dt} = k_1[A]](2/152e5e3c177fc5a95759f2380d916636.png)
![\frac{d[D]}{dt} = k_2[B] = k_2K[A]](f/d4fdbc4c253319ea30c6804f1d7926d1.png)
![\frac{\frac{d[D]}{dt}}{\frac{d[C]}{dt}}
= \frac{k_2K[A]}{k_1[A]}
= \frac{k_2K}{k_1}](2/7622d64d14cd4401d2d1d32cb254d19a.png)
![\frac{[D]}{[C]} = \frac{k_2K}{k_1}
= \frac{e^{-\Delta G_2^{\ddagger}/RT} e^{\Delta G/RT}}{e^{-\Delta G_1^{\ddagger}/RT}}
= e^{ - \frac{\Delta \Delta G^{\ddagger}}{RT} }](e/03e280de44333eb3d496902b8ef5b03f.png)