- Stereoisomerism
-
Stereoisomers are isomeric molecules that have the same molecular formula and sequence of bonded atoms (constitution), but that differ only in the three-dimensional orientations of their atoms in space.[1][2] This contrasts with structural isomers, which share the same molecular formula, but the bond connections and/or their order differ(s) between different atoms/groups. In stereoisomers, the order and bond connections of the constituent atoms remain the same, but their orientation in space differ.
Contents
Enantiomers
Main articles: Chirality (chemistry) and EnantiomerEnantiomers are two stereoisomers that are related to each other by a reflection: They are mirror images of each other, which are non-superimposable. Human hands are a macroscopic example of stereoisomerism. Every stereogenic center in one has the opposite configuration in the other. Two compounds that are enantiomers of each other have the same physical properties, except for the direction in which they rotate polarized light and how they interact with different optical isomers of other compounds. As a result, different enantiomers of a compound may have substantially different biological effects. Pure enantiomers also exhibit the phenomenon of optical activity and can be separated only with the use of a chiral agent. In nature, only one enantiomer of most chiral biological compounds, such as amino acids (except glycine, which is achiral), is present.
Diastereomers
Main article: DiastereomerDiastereomers are stereoisomers not related through a reflection operation. They are not mirror images of each other. These include meso compounds, cis-trans (E-Z) isomers, and non-enantiomeric optical isomers. Diastereomers seldom have the same physical properties. In the example shown below, the meso form of tartaric acid forms a diastereomeric pair with both levo and dextro tartaric acids, which form an enantiomeric pair.


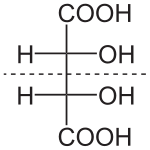
(natural) tartaric acid
L-(+)-tartaric acid
dextrotartaric acidD-(-)-tartaric acid
levotartaric acidmesotartaric acid
(1:1)
DL-tartaric acid
"racemic acid"It should be carefully noted here that the D- and L- labeling of the isomers above is not the same as the d- and l- labeling more commonly seen, explaining why these may appear reversed to those familiar with only the latter naming convention. Please refer to Chirality for more information regarding the D- and L- labels.
Cis-trans and E-Z isomerism
Main articles: Cis-trans isomerism and E-Z notationStereoisomerism about double bonds arises because rotation about the double bond is restricted, keeping the substituents fixed relative to each other. If the substituents on either end of a double bond are the same, it is not considered a stereo bond.
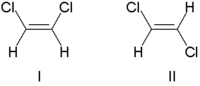
Traditionally, double bond stereochemistry was described as either cis (Latin, on this side) or trans (Latin, across), in reference to the relative position of substituents on either side of a double bond. The simplest examples of cis-trans isomerism are the 1,2-disubstituted ethenes, like the dichloroethene (C2H2Cl2) isomers shown below.
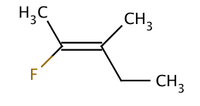
Molecule I is cis-1,2-dichloroethene and molecule II is trans-1,2-dichloroethene. Due to occasional ambiguity, IUPAC adopted a more rigorous system wherein the substituents at each end of the double bond are assigned priority based on their atomic number. If the high-priority substituents are on the same side of the bond, it is assigned Z (Ger. zusammen, together). If they are on opposite sides, it is E (Ger. entgegen, opposite). Since chlorine has a larger atomic number than hydrogen, it is the highest-priority group. Using this notation to name the above pictured molecules, molecule I is (Z)-1,2-dichloroethene and molecule II is (E)-1,2-dichloroethene. It is not the case that Z and cis or E and trans are always interchangeable. Consider the following fluoromethylpentene:
The proper name for this molecule is either trans-2-fluoro-3-methylpent-2-ene because the alkyl groups that form the backbone chain (i.e., methyl and ethyl) reside across the double bond from each other, or (Z)-2-fluoro-3-methylpent-2-ene because the highest-priority groups on each side of the double bond are on the same side of the double bond. Fluoro is the highest-priority group on the left side of the double bond, and ethyl is the highest-priority group on the right side of the molecule.
The terms cis and trans are also used to describe the relative position of two substituents on a ring; cis if on the same side, otherwise trans.
Conformers
Main article: Conformational isomerismConformational isomerism is a form of isomerism that describes the phenomenon of molecules with the same structural formula but with different shapes due to rotations about one or more bonds. Different conformations can have different energies, can usually interconvert, and are very rarely isolatable. For example, cyclohexane can exist in a variety of different conformations including a chair conformation and a boat conformation, but, for cyclohexane itself, these can never be separated. The boat conformation represents an energy maximum (and not a transition state) on the conformational itinerary between the two equivalent chair forms. There are some molecules that can be isolated in several conformations, due to the large energy barriers between different conformations. 2,2,2',2'-Tetrasubstituted biphenyls can fit into this latter category.
More definitions
- A configurational stereoisomer is a stereoisomer of a reference molecule that has the opposite configuration at a stereocenter (e.g., R- vs S- or E- vs Z-). This means that configurational isomers can be interconverted only by breaking covalent bonds to the stereocenter, for example, by inverting the configurations of some or all of the stereocenters in a compound.
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "stereoisomerism".
- ^ Columbia Encyclopedia. "Stereoisomers" in Encyclopedia.com, n.l., 2005, Link, December 7th 2005.
Concepts in asymmetric synthesis Chirality types Chirality · Stereocenter · Planar chirality · Chiral ligand · Axial chirality · Supramolecular chirality · Inherent chiralityChiral molecules Stereoisomer · Enantiomer · Diastereomer · Meso compound · Enantiomeric excess · Diastereomeric excess ·Analysis Optical rotation · Chiral derivatizing agents · NMR spectroscopy of stereoisomers · Ultraviolet-visible spectroscopy of stereoisomersChiral resolution Recrystallization · Kinetic resolution · Chiral column chromatography · Diastereomeric recrystallizationReactions Categories:- Stereochemistry
- Isomerism
Wikimedia Foundation. 2010.