- Sulpiride
-
Sulpiride 
Systematic (IUPAC) name (±)-5-(aminosulfonyl)-N-[(1-ethylpyrrolidin-2-yl)methyl]-2-methoxybenzamide Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status ℞ Prescription only Routes Oral Pharmacokinetic data Bioavailability 25–35% Half-life 7 hours Identifiers CAS number 15676-16-1 ATC code N05AL01 N05AL07 PubChem CID 5355 DrugBank APRD00032 ChemSpider 5162 
UNII 7MNE9M8287 
KEGG D01226 
ChEMBL CHEMBL26 
Chemical data Formula C15H23N3O4S Mol. mass 341.427 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Sulpiride (sold as Meresa, Bosnyl, Dogmatil, Dolmatil, Eglonyl, Modal; as Espiride in South Africa) is a first generation, or typical antipsychotic drug of the benzamide class used mainly in the treatment of psychosis associated with schizophrenia and major depressive disorder. Sulpiride is more commonly used in Europe and Japan. Levosulpiride is its purified levo- isomer and is sold in India for similar purpose. So far it has not been approved in the United States and Canada. The drug has strong chemical and clinical similarities to the related antipsychotic amisulpride.
Contents
Uses and dosage
- Productive psychosis: treatment with rather high doses—in excess of 600 mg daily
- Long-term treatment of negative (unproductive) psychosis: in moderate doses (approx. 600 mg daily)
- Treatment of depression and vertigo: in low to moderate doses (50 to 200 mg daily)
- Levosulpiride has also been promoted as a gastroprokinetic agent
Pharmacology
Pharmacokinetics
Sulpiride is absorbed slowly from the gastrointestinal tract. Its oral bioavailability is only 25 to 35% with marked interindividual differences. The peak plasma concentration is reached 4.5 hours after oral dosing. The usual half-life is 6 to 8 hours. Ninety-two percent is excreted unchanged in the urine. Sulpiride is usually given in 2 or 3 divided doses.
Pharmacodynamics
Sulpiride is a selective antagonist at dopamine D2 and D3 receptors. This action dominates in doses exceeding 600 mg daily. In doses of 600 to 1,600 mg sulpiride shows mild sedating and antipsychotic activity. Its antipsychotic potency compared to chlorpromazine is only 0.2 (1/5). In low doses (in particular 50 to 200 mg daily) its prominent feature is antagonism of presynaptic inhibitory dopamine receptors accounting for some antidepressant activity and a stimulating effect. Therefore, it is in these doses used as a second line antidepressant. Additionally, it alleviates vertigo.
The benzamide neuroleptics (including sulpiride, amisulpride, and sultopride) have been shown to activate the endogenous gamma-hydroxybutyrate receptor in vivo at therapeutic concentrations.[1] Sulpiride was found in one study in rats to upregulate GHB receptors.[2] GHB has neuroleptic properties and it is believed binding to this receptor may contribute to the effects of these neuroleptics.
Side effects
Sulpiride has fewer extrapyramidal side effects (dystonia, parkinsonism, and akathisia) than many of the older antipsychotic medications.[3] Most of these do not seem to occur in a dose related manner. Other side effects occur infrequently (hypotension, rarely long-QT syndrome, dry mouth, sweating, nausea, activation or sedation, insomnia, allergic rash or pruritus). Isolated cases of the potentially life-threatening NMS (neuroleptic malignant syndrome) have been reported. Sulpiride should not be taken after 4 p.m. in order to avoid insomnia. The foremost problem with sulpiride is a strong stimulation of prolactin-secretion; whether this may contribute to the development of breast-cancer in women is currently not known.
- Levodopa : Sulpiride and levodopa have antagonistic effects.
- Alcohol : Sedation and hypotension may be potentiated.
- Antihypertensive agents : Hypotension may be potentiated (risk of postural collapse).
- Other central depressants : Increased sedation with negative impact on the capacity to drive or operate machinery.
Overdose
Sulpiride has a relatively low order of acute toxicity. Substantial amounts may cause severe but reversible dystonic crises with torticollis, protrusion of the tongue, and/or trismus. In some cases all the classical symptoms typical of severe Parkinson's Disease may be noted; in others, over-sedation/coma may occur. The treatment is largely symptomatic. Some or all extrapyramidal reactions may respond to the application of anticholinergic drugs such as biperiden or benztropine. All patients should be closely monitored for signs of long-QT syndrome and severe arrhythmias.
Contraindications and cautions
- Hypersensitivity to sulpiride
- Pre-existing breast cancer or other prolactin-dependent tumors
- Phaeochromocytoma
- Intoxication with other centrally active drugs
- Concomitant use of levodopa
- Caution : Pre-existing Parkinson's Disease
- Caution : Patients below 18 years of age (insufficient clinical data)
- Caution : Pre-existing severe heart disease/bradycardia, or hypokalemia (predisposing to long QT syndrome and severe arrhythmias)
- Caution : Patients with pre-existing epilepsy. Anticonvulsant therapy should be maintained.
Pregnancy and lactation
- Pregnancy: Animal studies did not reveal any embryotoxicity or fetotoxicity, nor did limited human experience. Due to insufficient human data, pregnant women should be treated with sulpiride only if strictly indicated. Additionally, the newborns of treated women should be monitored, because isolated cases of extrapyramidal side effects have been reported.
- Lactation: Sulpiride is found in the milk of lactating women. Since the consequences are unclear, women should not breastfeed during treatment.
Chemistry
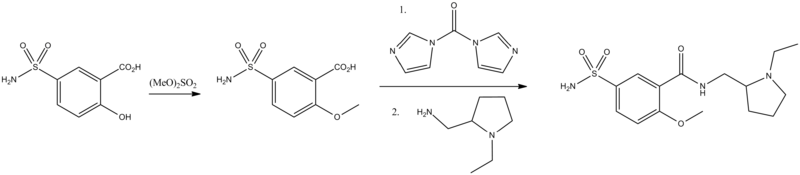
Sulpiride, (N-[(1-ethyl-2-pirrolidinylmethyl]-5-sulfamoyl-O-anizamide) is synthesized from 5-aminosulfosalicylic acid. Methylating this with dimethylsulfate gives 2-methoxy-5-aminosulfonylbenzoic acid, which is transformed into an amide using 2-aminomethyl-1-ethylpyrrolidine as the amine component and carbonyldiimidazole (CDI) as a condensing agent.
- E.L. Engelhardt, Ch.S. Miller, DE 1595915 (1965).
- E.L. Engelhardt, Ch.S. Miller, DE 1795723 (1965).
- E.L. Engelhardt, M.L. Thominet, U.S. Patent 3,342,826 (1969).
- G. Bulteau, J. Acher, U.S. Patent 4,077,976 (1978).
- F. Mauri, DE 2903891 (1979).
See also
References
- ^ Maitre M, Ratomponirina C, Gobaille S, Hodé Y, Hechler V (Apr 1994). "Displacement of [3H] gamma-hydroxybutyrate binding by benzamide neuroleptics and prochlorperazine but not by other antipsychotics". Eur J Pharmacol. 256 (2): 211–4. doi:10.1016/0014-2999(94)90248-8. PMID 7914168.
- ^ Ratomponirina C, Gobaille S, Hodé Y, Kemmel V, Maitre M (Apr 1998). "Sulpiride, but not haloperidol, up-regulates gamma-hydroxybutyrate receptors in vivo and in cultured cells". Eur J Pharmacol. 346 (2–3): 331–7. doi:10.1016/S0014-2999(98)00068-5. PMID 9652377. http://linkinghub.elsevier.com/retrieve/pii/S0014-2999(98)00068-5.
- ^ Sharpe, Michael; Harrison, Paul Carter; Geddes, John (2005). Lecture Notes: Psychiatry (Lecture Notes). Wiley-Blackwell. pp. 64. ISBN 1-4051-1869-5.
Dopaminergics Receptor ligands AgonistsAdamantanes: Amantadine • Memantine • Rimantadine; Aminotetralins: 7-OH-DPAT • 8-OH-PBZI • Rotigotine • UH-232; Benzazepines: 6-Br-APB • Fenoldopam • SKF-38,393 • SKF-77,434 • SKF-81,297 • SKF-82,958 • SKF-83,959; Ergolines: Bromocriptine • Cabergoline • Dihydroergocryptine • Lisuride • LSD • Pergolide; Dihydrexidine derivatives: 2-OH-NPA • A-86,929 • Ciladopa • Dihydrexidine • Dinapsoline • Dinoxyline • Doxanthrine; Others: A-68,930 • A-77,636 • A-412,997 • ABT-670 • ABT-724 • Aplindore • Apomorphine • Aripiprazole • Bifeprunox • BP-897 • CY-208,243 • Dizocilpine • Etilevodopa • Flibanserin • Ketamine • Melevodopa • Modafinil • Pardoprunox • Phencyclidine • PD-128,907 • PD-168,077 • PF-219,061 • Piribedil • Pramipexole • Propylnorapomorphine • Pukateine • Quinagolide • Quinelorane • Quinpirole • RDS-127 • Ro10-5824 • Ropinirole • Rotigotine • Roxindole • Salvinorin A • SKF-89,145 • Sumanirole • Terguride • Umespirone • WAY-100,635AntagonistsTypical antipsychotics: Acepromazine • Azaperone • Benperidol • Bromperidol • Clopenthixol • Chlorpromazine • Chlorprothixene • Droperidol • Flupentixol • Fluphenazine • Fluspirilene • Haloperidol • Loxapine • Mesoridazine • Methotrimeprazine • Nemonapride • Penfluridol • Perazine • Periciazine • Perphenazine • Pimozide • Prochlorperazine • Promazine • Sulforidazine • Sulpiride • Sultopride • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Trifluperidol • Zuclopenthixol; Atypical antipsychotics: Amisulpride • Asenapine • Blonanserin • Cariprazine • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Lurasidone • Melperone • Molindone • Mosapramine • Olanzapine • Paliperidone • Perospirone • Piquindone • Quetiapine • Remoxipride • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Antiemetics: AS-8112 • Alizapride • Bromopride • Clebopride • Domperidone • Metoclopramide • Thiethylperazine; Others: Amoxapine • Buspirone • Butaclamol • Ecopipam • EEDQ • Eticlopride • Fananserin • L-745,870 • Nafadotride • Nuciferine • PNU-99,194 • Raclopride • Sarizotan • SB-277,011-A • SCH-23,390 • SKF-83,959 • Sonepiprazole • Spiperone • Spiroxatrine • Stepholidine • Tetrahydropalmatine • Tiapride • UH-232 • YohimbineReuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugsGHBergics Receptor
ligands1,4-BD • 4-Methyl-GHB • GABOB • GBL • GHB • GHV • GVL • NCS-356 • NCS-435 • T-HCA • UMB66 • UMB68 • UMB72 • UMB86; Benzamides: Amisulpride • Sulpiride • SultoprideCategories:- Atypical antipsychotics
- Pyrrolidines
- Sulfonamides
- Benzamides
- Phenol ethers
Wikimedia Foundation. 2010.
