- Amineptine
-
Amineptine 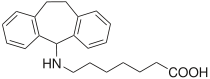
Amineptine chemical structure Systematic (IUPAC) name 7-[(10,11-dihydro-5H-dibenzo[a,d]-cyclohepten-5-yl)amino]heptanoic acid Clinical data Pregnancy cat. ? Legal status United Nations: Schedule II
Canada: Schedule III
USA: Unscheduled
UK: Schedule 2[1]
Germany: Anlage II (not prescribable)Routes Oral Pharmacokinetic data Bioavailability ? Metabolism Hepatic Half-life 48 mins (original drug)
2.5 hours (metabolites)[2]Excretion Renal Identifiers CAS number 57574-09-1 
ATC code N06AA19 PubChem CID 34870 DrugBank DB04836 ChemSpider 32091 
UNII 27T1I13L6G 
KEGG D07335 
ChEBI CHEBI:32499 
ChEMBL CHEMBL418995 
Chemical data Formula C22H28NO2 Mol. mass 337.455 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Amineptine was developed by the French Society of Medical research in the 1960s[3]. Under the trade-names (Survector, Maneon, Directim, Neolior, Provector, Viaspera) is used as an atypical tricyclic antidepressant (TCA) that selectively inhibits the reuptake of dopamine[4] and to a lesser extent norepinephrine, thus exerting a powerful and fast-acting antidepressant effect.[5]
Introduced in France in 1978 by the pharmaceutical giant Servier[6] and marketed under the trade name Survector, amineptine soon gained a reputation for abuse due to its short-lived, but pleasant, stimulant effect experienced by some patients. (This is to be distinguished from its antidepressant effect, which appears in approximately 7 days after commencing treatment.)
After its release into the European market, cases of hepatotoxicity emerged, some serious. This, along with the potential for abuse, led to the suspension of the French marketing authorization for Survector in 1999.
Amineptine was never approved by the U.S. Food and Drug Administration (FDA) for marketing in the United States, meaning that it is not legal to market or sell amineptine for any medical uses in the US.
Contents
Therapeutic indications
Approved
Amineptine was approved in France for severe clinical depression of endogenous origin in 1978.[7]
Unapproved/off-label/investigational
Parkinson's Disease, amotivational syndromes, ADHD (Attention Deficit/Hyperactivity Disorder)
Mechanism of action
- Inhibitor of the reuptake of norepinephrine and dopamine.
- Very weak muscarinic and histaminic receptors antagonist.
Side effects
Dermatological
Severe acne due to amineptine was first reported in 1988 by various authors—Grupper, Thioly-Bensoussan, Vexiau, Fiet, Puissant, Gourmel, Teillac, Levigne, to name a few—simultaneously[8][9][10][11][12] in the same issue of Annales de Dermatologie et de Venereologie and in the 12 March 1988 of The Lancet.[13] A year later, Dr Martin-Ortega and colleagues in Barcelona, Spain reported a case of "acneiform eruption" in a 54-year-old woman whose intake of amineptine was described as "excessive."[14] One year after that, Vexiau and colleagues reported six women, one of whom never admitted to using amineptine, getting severe acne concentrated in the face, back and thorax, the severity of which varied with the dosage.[15] Most of them were treated unsuccessfully with isotretinoin (Accutane) for about 18 months; two of the three that discontinued amineptine experienced a reduction in cutaneous symptoms, with the least affected patient going into remission.[15]
This can be seen as a general side effect of central dopamine enhancement, due to the inhibitory effect of dopamine on prolactin, with the subsequent increase in testosterone output, leading in turn to the same potential for acne as is typical of pubescents.
Psychiatric
Psychomotor excitation can very rarely occur with this drug.[16]
- Nervousness
- Irritability
- Insomnia
- Suicidal ideation. Seen early in the treatment, by lifting of psychomotor inhibition. This is a common occurrence with most, if not all, antidepressants.
Cardiovascular
Very rarely:
- Vasomotor episode
- Arterial hypotension
- Palpitations
Hepatic
Amineptine can rarely cause hepatitis, of the cytolytic, cholestatic varieties.[17] Amineptine-induced hepatitis, which is sometimes preceded by a rash, is believed to be due to an allergic reaction.[18] It resolves upon discontinuation of the offending drug.[17] The risk of getting this may or may not be genetically determined.[19]
Additionally, amineptine is known to rarely elevate transaminases, alkaline phosphatase, and bilirubin.[20]
Mixed hepatitis, which is very rare, generally occurs between the 15th and 30th day of treatment. Often preceded by sometimes intense abdominal pains, nausea, vomiting or a rash, the jaundice is variable. Hepatitis is either of mixed type or with cholestatic prevalence. The evolution was, in all the cases, favorable to the discontinuation of the drug. The mechanism is discussed (immunoallergic and/or toxic).[21]
In circa 1994 Spain, there was a case associating acute pancreatitis and mixed hepatitis, after three weeks of treatment.[22]
Lazaros and colleagues at the Western Attica General Hospital in Athens, Greece reported two cases of drug induced hepatitis 18 and 15 days of treatment.[23]
One case of cytolytic hepatitis occurred after ingestion of only one tablet.[24]
Gastrointestinal
- Acute pancreatitis (very rare) A case associating acute pancreatitis and mixed hepatitis after three weeks of treatment.[22]
Immunological
In 1989, Sgro and colleagues at the Centre de Pharmacovigilance in Dijon reported a case of anaphylactic shock in a woman who had been taking amineptine.[25]
Effects on the fetus
- Non-teratogenic in rodents
- Lacking information in humans
Abuse and dependence
The risk of addiction is low, but exists nonetheless. Between 1978 and 1988, there were 186 cases of amineptine addiction reported to the French Regional Centres of Pharmacovigilance; an analysis of 155 of those cases found that they were predominantly female, and that two-thirds of cases had known risk factors for addiction.[26] However, a 1981 study of known opiate addicts and schizophrenia patients found no drug addiction in any of the subjects.[27] In a 1990 study of eight amineptine dependence cases, the gradual withdrawal of amineptine could be achieved without problems in six people; in two others, anxiety, psychomotor agitation, and/or bulimia appeared.[28]
Precautions for use
- General anaesthesia: Discontinue the drug 24 to 48 hours before anaesthesia.
- Pregnancy (first trimester)
- Breast feeding
- Official sports/Olympic Games: Prohibited substance.
- 7 March Official Journal 2000.
Contraindications
- Chorea
- MAO inhibitors
- Children less than 1 year of age
- Hypersensitivity: Known hypersensitivity to amineptine, in particular antecedents of hepatitis after dosage of the product.
See also
References
- ^ http://www.nelm.nhs.uk/en/NeLM-Area/News/2011---March/29/New-Schedule-2-controlled-drugs--amineptine-and-tapentadol/
- ^ Lachatre G, Piva C, Riche C, et al. (1989). "Single-dose pharmacokinetics of amineptine and of its main metabolite in healthy young adults". Fundamental & Clinical Pharmacology 3 (1): 19–26. doi:10.1111/j.1472-8206.1989.tb00026.x. PMID 2714729.
- ^ DE Patent 2011806 - NEW TRICYCLIC DERIVATIVES AND PROCESS FOR THEIR MANUFACTURE
- ^ Vaugeois JM, Corera AT, Deslandes A, Costentin J (June 1999). "Although chemically related to amineptine, the antidepressant tianeptine is not a dopamine uptake inhibitor". Pharmacology, Biochemistry, and Behavior 63 (2): 285–90. doi:10.1016/S0091-3057(98)00242-1. PMID 10371658.
- ^ Wishart DS, Knox C, Guo AC, et al. (January 2006). "DrugBank: a comprehensive resource for in silico drug discovery and exploration". Nucleic Acids Research 34 (Database issue): D668–72. doi:10.1093/nar/gkj067. PMC 1347430. PMID 16381955. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1347430.
- ^ Sittig, Marshall (1988-04-01) [1979]. Pharmaceutical Manufacturing Encyclopedia (2nd ed.). Park Ridge, New Jersey, United States American: William Andrew Publishing/Noyes Publications. ISBN 0-8155-1144-2. http://www.amineptine.com/synthesis/manufacture.html. Retrieved 2005-10-29.[page needed]
- ^ Doctissimo (2005). "SURVECTOR - Amineptine" (in French). http://www.doctissimo.fr/medicament-SURVECTOR.htm. Retrieved 27 October 2005.
- ^ Grupper C (1988). "[New iatrogenic acne: acne caused by amineptin (Survector)]" (in French). Annales De Dermatologie et De Vénéréologie 115 (11): 1174–6. PMID 2977079.
- ^ Thioly-Bensoussan D, Charpentier A, Triller R, et al. (1988). "[Iatrogenic acne caused by amineptin (Survector). Apropos of 8 cases]" (in French). Annales De Dermatologie et De Vénéréologie 115 (11): 1177–80. PMID 2977080.
- ^ Vexiau P, Gourmel B, Husson C, et al. (1988). "[Severe lesions of acne type induced by chronic amineptin poisoning: apropos of 6 cases]" (in French). Annales De Dermatologie et De Vénéréologie 115 (11): 1180–2. PMID 2977081.
- ^ Teillac D, Weber MJ, Lowenstein W, de Prost Y (1988). "[Acne caused by Survector]" (in French). Annales De Dermatologie et De Vénéréologie 115 (11): 1183–4. PMID 2977082.
- ^ Lévigne V, Faisant M, Mourier C, et al. (1988). "[Monstrous acne in the adult. Inducer role of Survector?]" (in French). Annales De Dermatologie et De Vénéréologie 115 (11): 1184–5. PMID 2977083.
- ^ Vexiau P, Gourmel B, Julien R, et al. (March 1988). "Severe acne-like lesions caused by amineptine overdose". Lancet 1 (8585): 585. doi:10.1016/S0140-6736(88)91373-6. PMID 2894512.
- ^ Martín-Ortega E, Zamora E, Herrero C, Palou J (1989). "[Acneiform eruption induced by amineptin (Survector)]" (in Spanish). Medicina Cutánea Ibero-latino-americana 17 (6): 414–6. PMID 2534534.
- ^ a b Vexiau P, Gourmel B, Castot A, et al. (1990). "Severe acne due to chronic amineptine overdose". Archives of Dermatological Research 282 (2): 103–7. doi:10.1007/BF00493467. PMID 2141246.
- ^ "AMINEPTINE CHLORHYDRATE" (in French). BIAM. http://www.biam2.org/www/Gsu238.html. Retrieved 27 October 2005.
- ^ a b Bories P, Pomier-Layrargues G, Chotard JP, et al. (December 1980). "[Amineptine-induced cholestatic hepatitis. 5 cases (author's transl)]" (in French). La Nouvelle Presse Médicale 9 (48): 3689–92. PMID 7454584.
- ^ Pessayre D, Larrey D (April 1988). "Acute and chronic drug-induced hepatitis". Baillière's Clinical Gastroenterology 2 (2): 385–422. doi:10.1016/0950-3528(88)90009-7. PMID 3044468.
- ^ Larrey D, Pageaux GP (1997). "Genetic predisposition to drug-induced hepatotoxicity". Journal of Hepatology 26 (Suppl 2): 12–21. doi:10.1016/S0168-8278(97)80492-8. PMID 9204405.
- ^ "Questions au Professeur Daniel Dhumeaux [Drug-induced liver disorders. Questions for Professor Daniel Dhumeaux]" (in French). Gastroentérologie Clinique et Biologique 23 (8–9): 917–20. 1999. PMID 10533145. http://www.masson.fr/masson/MDOI-GCB-08-1999-23-8-9-0399-8320-101019-ART15.
- ^ Concours Med 1982; 104:5733-5734[verification needed]
- ^ a b Sebastián Domingo JJ, Simón Marco MA, Uribarrena Echebarría R (March 1994). "Hepatic and pancreatic injury associated with amineptine therapy". Journal of Clinical Gastroenterology 18 (2): 168–9. doi:10.1097/00004836-199403000-00023. PMID 8189020.
- ^ Lazaros GA, Stavrinos C, Papatheodoridis GV, Delladetsima JK, Toliopoulos A, Tassopoulos NC (1996). "Amineptine induced liver injury. Report of two cases and brief review of the literature". Hepato-gastroenterology 43 (10): 1015–9. PMID 8884331.
- ^ Jonville AP, Dutertre JP, Autret E (1992). "[Immediate acute hepatic cytolysis after the administration of a single amineptin tablet]" (in French). Gastroentérologie Clinique et Biologique 16 (4): 368. PMID 1397859.
- ^ Sgro C, Lacroix S, Waldner A, Lacroix M, Ferrut O, Bureau A (1989). "[Anaphylactic shock caused by amineptine. Report of a case]" (in French). La Revue De Médecine Interne 10 (5): 461–2. PMID 2488491.
- ^ Castot A, Benzaken C, Wagniart F, Efthymiou ML (1990). "[Amineptin abuse. Analysis of 155 cases. An evaluation of the official cooperative survey of the Regional Centers of Pharmacovigilance]" (in French). Thérapie 45 (5): 399–405. PMID 2260032.
- ^ Deniker P, Lôo H, Zarifian E, et al. (1981). "[Amineptine and amotival syndrome (author's transl)]" (in French). L'Encéphale 7 (1): 59–64. PMID 7227285.
- ^ Bertschy G, Luxembourger I, Bizouard P, Vandel S, Allers G, Volmat R (1990). "[Amineptin dependence. Detection of patients at risk. Report of 8 cases]" (in French). L'Encéphale 16 (5): 405–9. PMID 2265603.
External links
Stimulants (N06B) Adamantanes Adaphenoxate • Adapromine • Amantadine • Bromantane • Chlodantane • Gludantane • Memantine • Midantane
Adenosine antagonists 8-Chlorotheophylline • 8-Cyclopentyltheophylline • 8-Phenyltheophylline • Aminophylline • Caffeine • CGS-15943 • Dimethazan • Paraxanthine • SCH-58261 • Theobromine • TheophyllineAlkylamines Arylcyclohexylamines Benocyclidine • Dieticyclidine • Esketamine • Eticyclidine • Gacyclidine • Ketamine • Phencyclamine • Phencyclidine • Rolicyclidine • Tenocyclidine • Tiletamine
Benzazepines 6-Br-APB • SKF-77434 • SKF-81297 • SKF-82958
Cholinergics A-84543 • A-366,833 • ABT-202 • ABT-418 • AR-R17779 • Altinicline • Anabasine • Arecoline • Cotinine • Cytisine • Dianicline • Epibatidine • Epiboxidine • GTS-21 • Ispronicline • Nicotine • PHA-543,613 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • SIB-1553A • SSR-180,711 • TC-1698 • TC-1827 • TC-2216 • TC-5619 • Tebanicline • UB-165 • Varenicline • WAY-317,538
Convulsants Anatoxin-a • Bicuculline • DMCM • Flurothyl • Gabazine • Pentetrazol • Picrotoxin • Strychnine • Thujone
Eugeroics Adrafinil • Armodafinil • CRL-40941 • Modafinil
Oxazolines 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone
Phenethylamines 1-(4-Methylphenyl)-2-aminobutane • 1-Phenyl-2-(piperidin-1-yl)pentan-3-one • 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane • 2-Fluoroamphetamine • 2-Fluoromethamphetamine • 2-OH-PEA • 2-Phenyl-3-aminobutane • 2-Phenyl-3-methylaminobutane • 2,3-MDA • 3-Fluoroamphetamine • 3-Fluoroethamphetamine • 3-Fluoromethcathinone • 3-Methoxyamphetamine • 3-Methylamphetamine • 3,4-DMMC • 4-BMC • 4-Ethylamphetamine • 4-FA • 4-FMA • 4-MA • 4-MMA • 4-MTA • 6-FNE • Alfetamine • α-Ethylphenethylamine • Amfecloral • Amfepentorex • Amfepramone • Amidephrine • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Arbutamine • β-Methylphenethylamine • β-Phenylmethamphetamine • Benfluorex • Benzedrone • Benzphetamine • BDB (J) • BOH (Hydroxy-J) • BPAP • Buphedrone • Bupropion (Amfebutamone) • Butylone • Cathine • Cathinone • Chlorphentermine • Cinnamedrine • Clenbuterol • Clobenzorex • Cloforex • Clortermine • D-Deprenyl • Denopamine • Dimethoxyamphetamine • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Dobutamine • DOPA (Dextrodopa, Levodopa) • Dopamine • Dopexamine • Droxidopa • EBDB (Ethyl-J) • Ephedrine • Epinephrine (Adrenaline) • Epinine (Deoxyepinephrine) • Etafedrine • Ethcathinone (Ethylpropion) • Ethylamphetamine (Etilamfetamine) • Ethylnorepinephrine (Butanefrine) • Ethylone • Etilefrine • Famprofazone • Fenbutrazate • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenmetramide • Fenproporex • Flephedrone • Fludorex • Furfenorex • Gepefrine • HMMA • Hordenine • Ibopamine • IMP • Indanylamphetamine • Isoetarine • Isoethcathinone • Isoprenaline (Isoproterenol) • L-Deprenyl (Selegiline) • Lefetamine • Lisdexamfetamine • Lophophine (Homomyristicylamine) • Manifaxine • MBDB (Methyl-J; "Eden") • MDA (Tenamfetamine) • MDBU • MDEA ("Eve") • MDMA ("Ecstasy", "Adam") • MDMPEA (Homarylamine) • MDOH • MDPR • MDPEA (Homopiperonylamine) • Mefenorex • Mephedrone • Mephentermine • Metanephrine • Metaraminol • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methoxamine • Methoxyphenamine • MMA • Methcathinone (Methylpropion) • Methedrone • Methoxyphenamine • Methylone • MMDA • MMDMA • MMMA • Morazone • N-Benzyl-1-phenethylamine • N,N-Dimethylphenethylamine • Naphthylamphetamine • Nisoxetine • Norepinephrine (Noradrenaline) • Norfenefrine • Norfenfluramine • Normetanephrine • Octopamine • Orciprenaline • Ortetamine • Oxilofrine • Paredrine (Norpholedrine, Oxamphetamine, Mycadrine) • PBA • PCA • PHA • Pargyline • Pentorex (Phenpentermine) • Pentylone • Phendimetrazine • Phenmetrazine • Phenpromethamine • Phentermine • Phenylalanine • Phenylephrine (Neosynephrine) • Phenylpropanolamine • Pholedrine • PIA • PMA • PMEA • PMMA • PPAP • Prenylamine • Propylamphetamine • Pseudoephedrine • Radafaxine • Ropinirole • Salbutamol (Albuterol; Levosalbutamol) • Sibutramine • Synephrine (Oxedrine) • Theodrenaline • Tiflorex (Flutiorex) • Tranylcypromine • Tyramine • Tyrosine • Xamoterol • Xylopropamine • Zylofuramine
Piperazines Piperidines 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine • 1-(3,4-Dichlorophenyl)-1-(piperidin-2-yl)butane • 2-Benzylpiperidine • 2-Methyl-3-phenylpiperidine • 3,4-Dichloromethylphenidate • 4-Benzylpiperidine • 4-Methylmethylphenidate • Desoxypipradrol • Difemetorex • Diphenylpyraline • Ethylphenidate • Methylnaphthidate • Methylphenidate (Dexmethylphenidate) • N-Methyl-3β-propyl-4β-(4-chlorophenyl)piperidine • Nocaine • Phacetoperane • Pipradrol • SCH-5472
Pyrrolidines 2-Diphenylmethylpyrrolidine • α-PPP • α-PBP • α-PVP • Diphenylprolinol • MDPPP • MDPBP • MDPV • MPBP • MPHP • MPPP • MOPPP • Naphyrone • PEP • Prolintane • Pyrovalerone
Tropanes 3-CPMT • 3'-Chloro-3α-(diphenylmethoxy)tropane • 3-Pseudotropyl-4-fluorobenzoate • 4'-Fluorococaine • AHN-1055 • Altropane (IACFT) • Brasofensine • CFT (WIN 35,428) • β-CIT (RTI-55) • Cocaethylene • Cocaine • Dichloropane (RTI-111) • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Norcocaine • PIT • PTT • RTI-31 • RTI-32 • RTI-51 • RTI-105 • RTI-112 • RTI-113 • RTI-117 • RTI-120 • RTI-121 (IPCIT) • RTI-126 • RTI-150 • RTI-154 • RTI-171 • RTI-177 • RTI-183 • RTI-193 • RTI-194 • RTI-199 • RTI-202 • RTI-204 • RTI-229 • RTI-241 • RTI-336 • RTI-354 • RTI-371 • RTI-386 • Salicylmethylecgonine • Tesofensine • Troparil (β-CPT, WIN 35,065-2) • Tropoxane • WF-23 • WF-33 • WF-60
Others 1-(Thiophen-2-yl)-2-aminopropane • 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-MDP • 2-Phenylcyclohexylamine • 2-Phenyl-3,6-dimethylmorpholine • 3-Benzhydrylmorpholine • 3,3-Diphenylcyclobutanamine • 5-(2-Aminopropyl)indole • 5-Iodo-2-aminoindane • AL-1095 • Amfonelic acid • Amineptine • Amiphenazole • Atipamezole • Atomoxetine (Tomoxetine) • Bemegride • Benzydamine • BTQ • BTS 74,398 • Carphedon • Ciclazindol • Cilobamine • Clofenciclan • Cropropamide • Crotetamide • Cypenamine • D-161 • Diclofensine • Dimethocaine • Efaroxan • Etamivan • EXP-561 • Fencamfamine • Fenpentadiol • Feprosidnine • G-130 • Gamfexine • Gilutensin • GSK1360707F • GYKI-52895 • Hexacyclonate • Idazoxan • Indanorex • Indatraline • JNJ-7925476 • JZ-IV-10 • Lazabemide • Leptacline • Levopropylhexedrine • Lomevactone • LR-5182 • Mazindol • Meclofenoxate • Medifoxamine • Mefexamide • Mesocarb • Methastyridone • Methiopropamine • N-Methyl-3-phenylnorbornan-2-amine • Nefopam • Nikethamide • Nomifensine • O-2172 • Oxaprotiline • Phthalimidopropiophenone • PNU-99,194 • Propylhexedrine • PRC200-SS • Rasagiline • Rauwolscine • Rubidium chloride • Setazindol • Tametraline • Tandamine • Trazium • UH-232 • Yohimbine
See also Sympathomimetic aminesAntidepressants (N06A) Specific reuptake inhibitors (RIs), enhancers (REs), and releasing agents (RAs) Alaproclate • Citalopram • Escitalopram • Femoxetine • Fluoxetine# • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • Seproxetine • Sertraline# • Vilazodone • Zimelidine‡Bicifadine • Clovoxamine • Desvenlafaxine • Duloxetine • Levomilnacipran • Eclanamine • Milnacipran • Sibutramine • VenlafaxineSerotonin–norepinephrine–dopamine reuptake inhibitors (SNDRIs)Brasofensine • BTS-74,398 • Cocaine • Diclofensine • DOV-21,947 • DOV-102,677 • DOV-216,303 • EXP-561 • Fezolamine • JNJ-7925476 • NS-2359 • PRC200-SS • Pridefine • SEP-225,289 • SEP-227,162 • TesofensineAmedalin • Atomoxetine/Tomoxetine • Binedaline • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • ViloxazineDopamine reuptake inhibitors (DRIs)Amineptine • Bupropion/Amfebutamone# • Cilobamine • Manifaxine • Methylphenidate • Nomifensine • Radafaxine • TametralineNorepinephrine-dopamine releasing agents (NDRAs)Serotonin-norepinephrine-dopamine releasing agents (SNDRAs)4-Methyl-αMT • αET/Etryptamine • αMT/MetryptamineOthersIndeloxazine • Teniloxazine • Tramadol • ViqualineReceptor antagonists and/or reuptake inhibitors Serotonin antagonists and reuptake inhibitors (SARIs)Serotonin modulators and stimulators (SMSs)VortioxetineTricyclic and tetracyclic antidepressants (TCAs/TeCAs) TricyclicsAmezepine • Amineptine • Amitriptyline# • Amitriptylinoxide • Azepindole • Butriptyline • Cianopramine • Clomipramine • Cotriptyline • Cyanodothiepin • Demexiptiline • Depramine/Balipramine • Desipramine • Dibenzepin • Dimetacrine • Dosulepin/Dothiepin • Doxepin • Enprazepine • Fluotracen • Hepzidine • Homopipramol • Imipramine • Imipraminoxide • Intriptyline • Iprindole • Ketipramine • Litracen • Lofepramine • Losindole • Mariptiline • Melitracen • Metapramine • Mezepine • Naranol • Nitroxazepine • Nortriptyline • Noxiptiline • Octriptyline • Opipramol • Pipofezine • Propizepine • Protriptyline • Quinupramine • Tampramine • Tianeptine • Tienopramine • Trimipramine;7-OH-Amoxapine • Amoxapine • Aptazapine • Azipramine • Ciclazindol • Ciclopramine • Esmirtazapine • Loxapine • Maprotiline • Mazindol • Mianserin • Mirtazapine • Oxaprotiline • Setiptiline/TeciptilineMonoamine oxidase inhibitors (MAOIs) NonselectiveIrreversible: Benmoxin • Echinopsidine • Iproclozide • Iproniazid • Isocarboxazid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Safrazine • Tranylcypromine; Reversible: Caroxazone • Paraxazone;MAOA-SelectiveIrreversible: Clorgiline; Reversible: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Esuperone • Harmala Alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima;MAOB-SelectiveIrreversible: Ladostigil • Mofegiline • Pargyline • Rasagiline • Selegiline; Reversible: Lazabemide • MilacemideAzapirones and other 5-HT1A receptor agonists Alnespirone • Aripiprazole • Befiradol • Buspirone • Eptapirone • Flesinoxan • Flibanserin • Gepirone • Ipsapirone • Oxaflozane • Tandospirone • Vilazodone • ZalospironeAdrenergics Receptor ligands Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Cannabivarin • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.βAgonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • XipranololReuptake inhibitors Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • melitracen • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Ginkgo biloba • Indeloxazine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Teniloxazine • Tramadol • ZiprasidoneReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-OH-PEA • 4-CAB • 4-FA • 4-FMA • 4-MA • 4-MMA • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Me-PEA • BDB • Benzphetamine • BOH • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • DMA • DMMA • EBDB • Ephedrine • Ethcathinone • Ethylamphetamine • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • IAP • IMP • L-Deprenyl (Selegiline) • Lisdexamfetamine • Lophophine • MBDB • MDA (Tenamfetamine) • MDEA • MDMA • MDMPEA • MDOH • MDPEA • Mefenorex • Mephedrone • Mephentermine • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methcathinone • Methedrone • Methylone • NAP • Ortetamine • Paredrine • pBA • pCA • Pentorex (Phenpentermine) • Phenethylamine • Pholedrine • Phenpromethamine • Phentermine • Phenylpropanolamine • pIA • Prenylamine • Propylamphetamine • Pseudoephedrine • Tiflorex • Tyramine • Xylopropamine • Zylofuramine; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • pFPP; Others: 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-Benzylpiperidine • 4-Benzylpiperidine • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanorex • Isometheptene • Methylhexanamine • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • TuaminoheptaneEnzyme inhibitors 3,4-DihydroxystyreneDBHCGS-19281A • SKF-64139 • SKF-7698Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline • Safinamide
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.COMTOthers Ferrous Iron (Fe2+) • S-Adenosyl-L-Methionine • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal Phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)List of adrenergic drugsDopaminergics Receptor ligands AgonistsAdamantanes: Amantadine • Memantine • Rimantadine; Aminotetralins: 7-OH-DPAT • 8-OH-PBZI • Rotigotine • UH-232; Benzazepines: 6-Br-APB • Fenoldopam • SKF-38,393 • SKF-77,434 • SKF-81,297 • SKF-82,958 • SKF-83,959; Ergolines: Bromocriptine • Cabergoline • Dihydroergocryptine • Lisuride • LSD • Pergolide; Dihydrexidine derivatives: 2-OH-NPA • A-86,929 • Ciladopa • Dihydrexidine • Dinapsoline • Dinoxyline • Doxanthrine; Others: A-68,930 • A-77,636 • A-412,997 • ABT-670 • ABT-724 • Aplindore • Apomorphine • Aripiprazole • Bifeprunox • BP-897 • CY-208,243 • Dizocilpine • Etilevodopa • Flibanserin • Ketamine • Melevodopa • Modafinil • Pardoprunox • Phencyclidine • PD-128,907 • PD-168,077 • PF-219,061 • Piribedil • Pramipexole • Propylnorapomorphine • Pukateine • Quinagolide • Quinelorane • Quinpirole • RDS-127 • Ro10-5824 • Ropinirole • Rotigotine • Roxindole • Salvinorin A • SKF-89,145 • Sumanirole • Terguride • Umespirone • WAY-100,635AntagonistsTypical antipsychotics: Acepromazine • Azaperone • Benperidol • Bromperidol • Clopenthixol • Chlorpromazine • Chlorprothixene • Droperidol • Flupentixol • Fluphenazine • Fluspirilene • Haloperidol • Loxapine • Mesoridazine • Methotrimeprazine • Nemonapride • Penfluridol • Perazine • Periciazine • Perphenazine • Pimozide • Prochlorperazine • Promazine • Sulforidazine • Sulpiride • Sultopride • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Trifluperidol • Zuclopenthixol; Atypical antipsychotics: Amisulpride • Asenapine • Blonanserin • Cariprazine • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Lurasidone • Melperone • Molindone • Mosapramine • Olanzapine • Paliperidone • Perospirone • Piquindone • Quetiapine • Remoxipride • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Antiemetics: AS-8112 • Alizapride • Bromopride • Clebopride • Domperidone • Metoclopramide • Thiethylperazine; Others: Amoxapine • Buspirone • Butaclamol • Ecopipam • EEDQ • Eticlopride • Fananserin • L-745,870 • Nafadotride • Nuciferine • PNU-99,194 • Raclopride • Sarizotan • SB-277,011-A • SCH-23,390 • SKF-83,959 • Sonepiprazole • Spiperone • Spiroxatrine • Stepholidine • Tetrahydropalmatine • Tiapride • UH-232 • YohimbineReuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugsTricyclics Classes Acridine • Anthracene • Dibenzazepine • Dibenzocycloheptene • Dibenzodiazepine • Dibenzothiazepine • Dibenzothiepin • Dibenzoxazepine • Dibenzoxepin • Phenothiazine • Pyridazinobenzoxazine • Pyridinobenzodiazepine • ThioxantheneAntidepressants 7-OH-Amoxapine • Amezepine • Amineptine • Amitriptyline • Amitriptylinoxide • Amoxapine • Aptazapine • Azepindole • Azipramine • Butriptyline • Cianopramine • Ciclazindol • Ciclopramine • Clomipramine • Cotriptyline • Cyanodothiepin • Demexiptiline • Depramine/Balipramine • Desipramine • Dibenzepin • Dimetacrine • Dosulepin/Dothiepin • Doxepin • Enprazepine • Esmirtazapine • Fluotracen • Hepzidine • Homopipramol • Imipramine • Imipraminoxide • Intriptyline • Iprindole • Ketipramine • Litracen • Lofepramine • Losindole • Loxapine • Maprotiline • Mariptiline • Mazindol • Melitracen • Metapramine • Mezepine • Mianserin • Mirtazapine • Naranol • Nitroxazepine • Nortriptyline • Noxiptiline • Octriptyline • Opipramol • Oxaprotiline • Pipofezine • Pirandamine • Propizepine • Protriptyline • Quinupramine • Setiptiline/Teciptiline • Tandamine • Tampramine • Tianeptine • Tienopramine • TrimipramineAntihistamines Alimemazine • Azatadine • Clobenzepam • Cyproheptadine • Dacemazine • Deptropine • Desloratadine • Epinastine • Etymemazine • Hydroxyethylpromethazine • Isopromethazine • Isothipendyl • Ketotifen • Latrepirdine • Loratadine • Mebhydrolin • Mequitazine • Methdilazine • Olopatadine • Oxomemazine • Phenindamine • Pimethixene • Promethazine • Propiomazine • Rupatadine • ThiazinamiumAntipsychotics Acetophenazine • Amoxapine • Asenapine • Butaclamol • Butaperazine • Carphenazine • Carpipramine • Chlorpromazine • Chlorprothixene • Ciclindole • Clocapramine • Clomacran • Clotiapine • Clozapine • Flucindole • Fluotracen • Flupentixol • Fluphenazine • Gevotroline • Homopipramol • Levomepromazine/Methotrimeprazine • Loxapine • Maroxepin • Mesoridazine • Metitepine/Methiothepin • Metoxepin • Mosapramine • Naranol • Olanzapine • Perazine • Perphenazine • Periciazine • Piperacetazine • Pipotiazine • Piquindone • Prochlorperazine • Promazine • Prothipendyl • Quetiapine • Sulforidazine • Thiethylperazine • Thiopropazate • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Zotepine • ZuclopenthixolOthers Atiprosin • Carbamazepine • Carvedilol • Cyclobenzaprine • Licarbazepine • Methylene Blue • Monatepil • Oxcarbazepine • Oxitriptyline • Pirenzepine • Pirolate • Pitrazepin • Pizotifen • ProfenamineCategories:- Tricyclic antidepressants
- Withdrawn drugs
- Dibenzocycloheptenes
- Servier
- Norepinephrine-dopamine reuptake inhibitors
Wikimedia Foundation. 2010.
