- Acetylide
-
Acetylide Identifiers ChemSpider 6113 
Jmol-3D images Image 1 - [C-]#[C-]
Properties Molecular formula C2−
2Molar mass 24.0214 Exact mass 24.000000000000 g mol-1 Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Acetylide, ethynide, dicarbide, or percarbide is the divalent anion with formula C22− or (C≡C)2−. It may be regarded as the result of removing two protons from acetylene C2H2 or H-C≡C-H, the prototypical alkyne — that behaves as a weak acid.
These terms are also used for any monovalent anion of the form R-C≡C−, where R is any monovalent organic moiety; such as hydrogenacetylide H-C≡C−, or methylacetylide H3C-C≡C−.
The anion names are also used for any salt containing them, such as copper acetylide (Cu+)2·C22−, lithium hydrogenacetylide Li+·HC2−, or silver methylacetylide. Ag+·(CH3)C2−. Some salts of the C22− anion are traditionally called carbides, e.g. calcium carbide Ca2+·C22− and lithium carbide (Li+)2·C22−.
Some acetylides are explosive, and their accidental formation is a major safety risk in acetylene processing or storage. Acetylides are very useful reagents in organic chemistry.
Contents
Synthesis
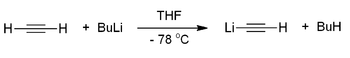
Acetylides of the alkali metals can be prepared by dissolving the metal in liquid ammonia and passing acetylene through the solution. Other strong bases such as butyllithium[1] or LiHMDS[2] are also frequently used:
Copper(I) acetylide can be prepared by passing acetylene through a water solution of copper(I) chloride. Silver acetylide can be obtained in a similar way from silver nitrate.
Calcium carbide is prepared by reacting carbon with lime CaO at approximately 2000 °C. A similar process is used to produce lithium carbide.
Reactions
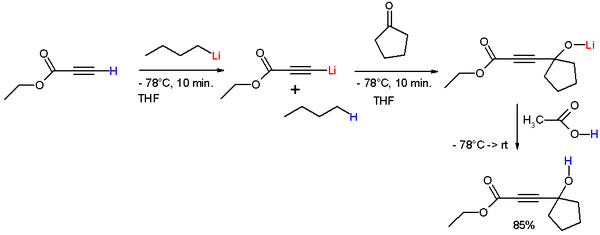
Acetylide ions are very useful in organic chemistry reactions in combining carbon chains, particularly addition and substitution reactions. One type of reaction displayed by acetylides are addition reactions with ketones to form tertiary alcohols. In the reaction in scheme 1 the alkyne proton of ethyl propiolate is deprotonated by n-butyllithium at -78°C to form lithium ethyl propiolate to which cyclopentanone is added forming a lithium alkoxide. Acetic acid is added to remove lithium and liberate the free alcohol.[3]
Coupling reactions of alkynes like the Sonogashira coupling, the Cadiot-Chodkiewicz coupling, the Glaser coupling and the Eglinton coupling often have metal acetylides as intermediates.
Several modifications of the reaction with carbonyls are known:
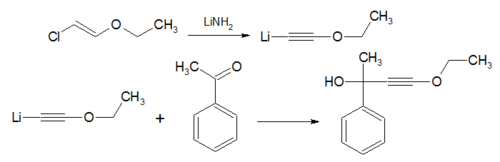
- In the Arens-van Dorp Synthesis the compound ethoxyacetylene [4] is converted to a Grignard reagent and reacted with a ketone, the reaction product is a propargyl alcohol.[5]
- In the Isler modification ethoxyacetylene is replaced by beta-chlorovinyl ether and lithium amide.
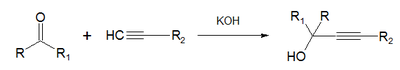
- In the Favorskii-Babayan synthesis ketones and acetylenic compounds react in presence of alkali.[6]
Formation of acetylides poses a risk in handling of gaseous acetylene in presence of metals such as mercury, silver or copper, or alloys with their high content (brass, bronze, silver solder).
See also
- Acetylenediol
References
- ^ Midland, M. M.; McLoughlin, J. I.; Werley, Ralph T. (Jr.) (1990), "Preparation and Use of Lithium Acetylide: 1-Methyl-2-ethynyl-endo-3,3-dimethyl-2-norbornanol", Org. Synth. 68: 14, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV8P0391; Coll. Vol. 8: 391
- ^ Reich, Melanie (Aug 24, 2001). "Addition of a lithium acetylide to an aldehyde; 1-(2-pentyn-4-ol)-cyclopent-2-en-1-ol". ChemSpider Synthetic Pages. p. 137. http://cssp.chemspider.com/137. Retrieved 5 September 2010.
- ^ Synthesis of alkyl 4-hydroxy-2-alkynoates M. Mark Midland, Alfonso Tramontano, John R. Cable J. Org. Chem.; 1980; 45(1); 28-29. Abstract
- ^ Organic Syntheses, Coll. Vol. 4, p.404 (1963); Vol. 34, p.46 (1954). Link
- ^ van Dorp and Arens, Nature, 160, 189 (1947).
- ^ Favorskii-Babayan Synthesis
Categories:- Ion pages needing a structure drawing
- Anions
- Functional groups
- Acetylides
Wikimedia Foundation. 2010.