- 2-Mercaptopyridine
-
2-Mercaptopyridine  Other names2-thiopyridine; 2-thiopyridone; pyrid-2-thione; 2-pyridyl mercaptan; 2-pyridinethiol; 2-pyridinethione
Other names2-thiopyridine; 2-thiopyridone; pyrid-2-thione; 2-pyridyl mercaptan; 2-pyridinethiol; 2-pyridinethioneIdentifiers CAS number 2637-34-5 
ChemSpider 2005897 
DrugBank DB03329 ChEBI CHEBI:45223 
ChEMBL CHEMBL1235541 
Jmol-3D images Image 1 - S=C1/C=C\C=C/N1
Properties Molecular formula C5H5NS Molar mass 111.16 g mol−1 Appearance yellow crystalline powder Melting point 128-130°C (400-403 K)
Solubility in water 50 g/L Hazards MSDS MSDS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
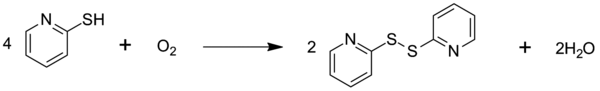
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 2-Mercaptopyridine is an organosulfur compound with the formula HSC5H4N. This yellow crystalline solid is a derivative of pyridine. The compound and its derivatives serve primarily as acylating agents. A few of 2-mercaptopyridine’s other uses include serving as a protecting group for amines and imides as well as forming a selective reducing agent. 2-Mercaptopyridine oxidizes to 2,2’-dipyridyl disulfide.[1]
Contents
Preparation
2-Mercaptopyridine was originally synthesized in 1931 by heating 2-chloropyridine with calcium hydrogen sulfide.[2]
- ClC5H4N + Ca(SH)2 → HSC5H4N + Ca(SH)Cl
A more convenient route to 2-mercaptopyridine is the reaction of 2-chloropyridine and thiourea in ethanol and aqueous ammonia.[3]
2-Mercaptopyridine derivatives can also be generated from precursors lacking preformed pyridine rings. It arises for example in the condensation of α,β-unsaturated ketones, malononitrile, and 4-methylbenzenethiol under microwave irradiation. The reaction is conducted with a base catalyst.[4]
Structure and properties
Similar in nature to 2-hydroxypyridine, 2-mercaptopyridine converts to the thione tautomer. The preferred form is dependent on temperature, concentration, and solvent. The thione is favored at lower temperatures, lower concentrations, and in less polar solvents.[5][6]
2-Mercaptopyridine oxidizes to 2,2’-dipyridyl disulfide. 2-Mercaptopyridine is favored in dilute solutions and in solvents capable of hydrogen bonding. These solvents will compete with other 2-mercaptopyridines to prevent self association.[6]
The association constant for this reaction between mutual 2-mercaptopyridines is described below. The ratio is of monosulfide to disulfide in chloroform.[6]
- Kassociation = (2.7±0.5)x103
2-Mercaptopyridine can also be prepared by hydride reduction of 2,2’-dipyridyl disulfide.
- C5H4NSSC5H4N + 2H → 2HSC5H4N
Main reactions
2-Mercaptopyridine and the disulfide are chelating ligands. 2-mercaptopyridine forms the indium(III) complex In(PyS)3 complexes in supercritical carbon dioxide.[7] 2-Mercaptopyridine may also be used to coat porous media in order to purify plasmid DNA of impurities such as RNA and proteins at relatively quick timescales to similar methods.[8] 2-Mercaptopyridine is also used acylate phenols, amines, and carboxylic acids.[1]
References
- ^ a b Adams, Edward J.; Skrydstrup, Troels; Lindsay, Karl B.; Skrydstrup, Troels; Lindsay, Karl B. (2007). 2-Pyridinethiol. doi:10.1002/047084289X.rp286.pub3.
- ^ Räth, C.; Binz, A.; Räth, C. (1931). "Mercaptane und Sulfosäuren des Pyridins. XII. Mitteilung über Derivate des Pyridins". Justus Liebig's Annalen der Chemie 487: 105. doi:10.1002/jlac.19314870107.
- ^ Jones, R. A.; Katritzky, A. R. (1958). "721. Tautomeric pyridines. Part I. Pyrid-2- and -4-thione". Journal of the Chemical Society (Resumed): 3610. doi:10.1039/JR9580003610.
- ^ Wang, Xing-Han; Cao, Xu-Dong; Tu, Shu-Jiang; Zhang, Xiao-Hong; Hao, Wen-Juan; Yan, Shu; Wu, Shan-Shan; Han, Zheng-Guo et al. (2009). "An efficient and direct synthesis of 2-thiopyridinesviamicrowave-assisted three-component reaction". Journal of Heterocyclic Chemistry 46 (5): 886. doi:10.1002/jhet.161.
- ^ Moran, Damian; Sukcharoenphon, Kengkaj; Puchta, Ralph; Schaefer, Henry F.; Schleyer, Paul v. R.; Hoff, Carl D. (2002). "2-Pyridinethiol/2-Pyridinethione Tautomeric Equilibrium. A Comparative Experimental and Computational Study". The Journal of Organic Chemistry 67 (25): 9061. doi:10.1021/jo0263768. PMID 12467429.
- ^ a b c Beak, Peter; Covington, Johnny B.; Smith, Stanley G.; White, J. Matthew; Zeigler, John M. (1980). "Displacement of protomeric equilibriums by self-association: hydroxypyridine-pyridone and mercaptopyridine-thiopyridone isomer pairs". The Journal of Organic Chemistry 45 (8): 1354. doi:10.1021/jo01296a002.
- ^ Chou, Wei-Lung; Yang, Kai-Chiang (2008). "Effect of various chelating agents on supercritical carbon dioxide extraction of indium(III) ions from acidic aqueous solution". Journal of Hazardous Materials 154 (1–3): 498. doi:10.1016/j.jhazmat.2007.10.052. PMID 18054158.
- ^ Li, Yuan; Dong, Xiao-Yan; Sun, Yan (2007). "Biporous polymeric microspheres coupled with mercaptopyridine for rapid chromatographic purification of plasmid DNA". Journal of Applied Polymer Science 104 (4): 2205. doi:10.1002/app.24417.
Categories:- Pyridines
- Thiols
Wikimedia Foundation. 2010.