- Light-dependent reactions
-
See also: Light-independent reactions
The 'light-dependent reactions', or light reactions, are the first stage of photosynthesis, the process by which plants capture and store energy from sunlight. In this process, light energy is converted into chemical energy, in the form of the energy-carrying molecules ATP and NADPH. In the light-independent reactions, the formed NADPH and ATP drive the reduction of CO2 to more useful organic compounds, such as glucose. However, although light-independent reactions are, by convention, also called dark reactions, they are not independent of the need of light, for they are driven by ATP and NADPH, products of light.
The light-dependent reactions take place on the thylakoid membrane inside a chloroplast. The inside of the thylakoid membrane is called the lumen, and outside the thylakoid membrane is the stroma, where the light-independent reactions take place. The thylakoid membrane contains some integral membrane protein complexes that catalyze the light reactions. There are four major protein complexes in the thylakoid membrane: Photosystem I (PSI), Photosystem II (PSII), Cytochrome b6f complex, and ATP synthase. These four complexes work together to ultimately create the products ATP and NADPH.
The two photosystems absorb light energy through proteins containing pigments, such as chlorophyll. The light-dependent reactions begin in photosystem II. When a chlorophyll a molecule within the reaction center of PSII absorbs a photon, an electron in this molecule attains a higher energy level. Because this state of an electron is very unstable, the electron is transferred from one to another molecule creating a chain of redox reactions, called an electron transport chain (ETC). The electron flow goes from PSII to cytochrome b6f to PSI. In PSI, the electron gets the energy from another photon. The final electron acceptor is NADP. In oxygenic photosynthesis, the first electron donor is water, creating oxygen as a waste product. In anoxygenic photosynthesis various electron donors are used.
Cytochrome b6f and ATP synthase work together to create ATP. This process is called photophosphorylation, which occurs in two different ways. In non-cyclic photophosphorylation, cytochrome b6f uses the energy of electrons from PSII to pump protons from the stroma to the lumen. The proton gradient across the thylakoid membrane creates a proton-motive force, used by ATP synthase to form ATP. In cyclic photophosphorylation, cytochrome b6f uses the energy of electrons from not only PSII but also PSI to create more ATP and to stop the production of NADPH. Cyclic phosphorylation is important to create ATP and maintain NADPH in the right proportion for the light-independent reactions.
The net-reaction of all light-dependent reactions in oxygenic photosynthesis is:
2H2O + 2NADP+ + 3ADP + 3Pi → O2 + 2NADPH + 3ATPThe two photosystems are protein complexes that absorb photons and are able to use this energy to create an electron transport chain. Photosystem I and II are very similar in structure and function. They use special proteins, called light-harvesting complexes, to absorb the photons with very high effectiveness. If a special pigment molecule in a photosynthetic reaction center absorbs a photon, an electron in this pigment attains the excited state and then is transferred to another molecule in the reaction center. This reaction, called photoinduced charge separation, is the start of the electron flow and is unique because it transforms light energy into chemical forms.
Contents
The light-harvesting system
A common misconception is that photosynthesis relies only on chlorophyll pigments. The truth is that photosynthesis would be rather inefficient using only chlorophyll molecules. Chlorophyll molecules absorb light only at specific wavelengths (see image). A large gap is present in the middle of the visible regions between approximately 450 and 650 nm. This gap corresponds to the peak of the solar spectrum, so failure to collect this light would constitute a considerable lost opportunity. That's why photosynthesis organisms have developed a light-harvesting system, which bundles different pigments to create a much wider absorption spectrum.
The light harvesting-system is composed of numerous light-harvesting complexes that completely surround the reaction center where the Photoinduced charge separation takes place. Chlorophyll, carotenes, and xanthophylls are arranged in such light-harvesting complexes or LHC-proteins. These pigments are referred to as accessory pigments and funnel the energy to a special pigment in the reaction center of PSI or PSII.
If a pigment molecule absorbs a photon, an electron in the molecule becomes excited. For most compounds that absorb light, the electron simply returns to the ground state and the absorbed energy is converted into heat and/or fluorescence. But, in a LHC-protein, the pigments are so arranged that the excitation energy can be transferred from one molecule to a nearby molecule. The rate of this process, called resonance energy transfer, depends strongly on the distance between the energy donor and energy acceptor molecules. For reasons of conservation of energy, energy transfer must be from a donor in the excited state to an acceptor of equal or lower energy. If the energy of a photon becomes lower, the wavelength also becomes longer. When chlorophyll is isolated from the enzymes it is associated with, the second scenario can be seen to happen. The pigments in an LHC-protein are so arranged that pigments are very close to each other and a pigment is near another pigment that absorbs photons with a longer wavelength. As a consequence, the pigment in the reaction center has to absorb photons with the longest wavelength and cannot transfer this energy to another pigment. The function of LHC-proteins is to create a constant supply of excitation-energy to the reaction center pigment. Every reaction center has a couple of LHC-proteins.
The reaction center
Main article: Photosynthetic reaction centre(chlorophyll)
The reaction center is in the thylakoid membrane. It transfers light energy to a dimer of chlorophyll pigment molecules near the periplasmic (or thylakoid lumen) side of the membrane. This dimer is called a special pair because of its fundamental role in photosynthesis. This special pair is slightly different in PSI and PSII reaction center. In PSII, it absorbs photons with a wavelength of 680 nm, and it is therefore called P680. In PSI, it absorbs photons at 700 nm, and it is called P700. In bacteria, the special pair is called P760, P840, P870, or P960.
If an electron of the special pair in the reaction center becomes excited, it cannot transfer this energy to another pigment using resonance energy transfer. In normal circumstances, the electron should return to the ground state, but, because the reaction center is so arranged that a suitable electron acceptor is nearby, the excited electron can move from the initial molecule to the acceptor. This process results in the formation of a positive charge on the special pair (due to the loss of an electron) and a negative charge on the acceptor and is, hence, referred to as photoinduced charge separation. In other words, electrons in pigment molecules can exist at specific energy levels. Under normal circumstances, they exist at the lowest possible energy level they can. However, if there is enough energy to move them into the next energy level, they can absorb that energy and occupy that higher energy level. The light they absorb contains the necessary amount of energy needed to push them into the next level. Any light that does not have enough or has too much energy cannot be absorbed and is reflected. The electron in the higher energy level, however, does not want to be there; the electron is unstable and must return to its normal lower energy level. To do this, it must release the energy that has put it into the higher energy state to begin with. This can happen various ways. The extra energy can be converted into molecular motion and lost as heat. Some of the extra energy can be lost as heat energy, while the rest is lost as light. This re-emission of light energy is called florescence. The energy, but not the e- itself, can be passed onto another molecule. This is called resonance. The energy and the e- can be transferred to another molecule. Plant pigments usually utilize the last two of these reactions to convert the sun's energy into their own.
This initial charge separation occurs in less than 10 picoseconds (10−11 seconds). In their high-energy states, the special pigment and the acceptor could undergo charge recombination; that is, the electron on the acceptor could move back to neutralize the positive charge on the special pair. Its return to the special pair would waste a valuable high-energy electron and simply convert the absorbed light energy into heat. Three factors in the structure of the reaction center work together to suppress charge recombination nearly completely.
- Another electron acceptor is less than 10 Å away from the first acceptor, and so the electron is rapidly transferred farther away from the special pair.
- An electron donor is less than 10 Å away from the special pair, and so the positive charge is neutralized by the transfer of another electron
- The electron transfer from the electron acceptor to the positively charged special pair is especially slow: The transfer is so thermodynamically favorable that it takes place in the inverted region where electron-transfer rates become slower.
Thus, electron transfer proceeds efficiently from the first electron acceptor to the next, creating an electron transport chain that ends if it has reached NADPH.
Photosynthetic electron transport chains in chloroplasts
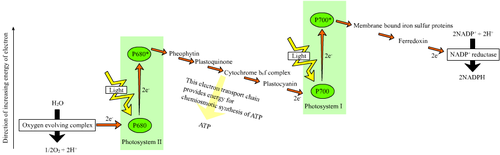
The photosynthesis process in chloroplasts begins when an electron of P680 of PSII attains an higher-energy level. This energy is used to reduce a chain of electron acceptors that have subsequently lowered redox-potentials. This chain of electron acceptors is known as an electron transport chain. When this chain reaches PS I, an electron is again excited, creating a high redox-potential. The electron transport chain of photosynthesis is often put in a diagram called the z-scheme, because the redox diagram from P680 to P700 resembles the letter z.[1]
The final product of PSII is plastoquinol, a mobile electron carrier in the membrane. Plastoquinol transfers the electron from PSII to the proton pump, cytochrome b6f. The ultimate electron donor of PSII is water. Cytochrome b6f proceeds the electron chain to PSI through plastocyanin molecules. PSI is able to continue the electron transfer in two different ways. It can transfer the electrons either to plastoquinol again, creating a cyclic electron flow, or to an enzyme called FNR, creating a non-cyclic electron flow. PSI releases FNR into the stroma, where it reduces NADP+ to NADPH.
Activities of the electron transport chain, especially from cytochrome b6f, lead to pumping of protons from the stroma to the lumen. The resulting transmembrane proton gradient is used to make ATP via ATP synthase.
The overall process of the photosynthetic electron transport chain in chloroplasts is:
H2O → PS II → plastoquinone → cytb6f → plastocyanin → PS I → NADPH Photosystem II
Main article: Photosystem IIPS II is an extremely complex, highly organized transmembrane structure that contains a water-splitting complex, chlorophylls and carotenoid pigments, a reaction center (P680), pheophytin (a pigment similar to chlorophyll), and two quinones. It uses the energy of sunlight to transfer electrons from water to a mobile electron carrier in the membrane called plastoquinone:
H2O → P680 → P680* → plastoquinone Plastoquinone, in turn, transfers electrons to b6f, which feeds them into PS I.
The water-splitting complex
The step H2O → P680 is performed by a poorly-understood structure embedded within PS II called the water-splitting complex or the oxygen-evolving complex. It catalyzes a reaction that splits water into electrons, protons and oxygen:
2H2O → 4H+ + 4e- + O2 The electrons are transferred to special chlorophyll molecules (embedded in PS II) that are promoted to a higher-energy state by the energy of photons.
The reaction center
The excitation P680 → P680*of the reaction center pigment P680 occurs here. These special chlorophyll molecules embedded in PS II absorb the energy of photons, with maximal absorption at 680 nm. Electrons within these molecules are promoted to a higher-energy state. This is one of two core processes in photosynthesis, and it occurs with astonishing efficiency (greater than 90%) because, in addition to direct excitation by light at 680 nm, the energy of light first harvested by antenna proteins at other wavelengths in the light-harvesting system is also transferred to these special chlorophyll molecules.
This is followed by the step P680*→ pheophytin, and then on to plastoquinone, which occurs within the reaction center of PS II. High-energy electrons are transferred to plastoquinone. Plastoquinone is then released into the membrane as a mobile electron carrier.
This is the second core process in photosynthesis. The initial stages occur within picoseconds, with an efficiency of 100%. The seemingly impossible efficiency is due to the precise positioning of molecules within the reaction center. This is a solid-state process, not a chemical reaction. It occurs within an essentially crystalline environment created by the macromolecular structure of PS II. The usual rules of chemistry (which involve random collisions and random energy distributions) do not apply in solid-state environments.
Link of water-splitting complex and chlorophyll excitation
When the chlorophyll passes the electron to pheophytin, it obtains an electron from P680*. In turn, P680* can oxidize the Z (or YZ) molecule. Once oxidized, the Z molecule can derive electrons from the water-splitting complex.[2]
Summary
PS II is a transmembrane structure found in all chloroplasts. It splits water into electrons, protons and molecular oxygen. The electrons are transferred to plastoquinone, which carries them to a proton pump. Molecular oxygen is released into the atmosphere.
The emergence of such an incredibly complex structure, a macromolecule that converts the energy of sunlight into potentially useful work with efficiencies that are impossible in ordinary experience, seems almost magical at first glance. Thus, it is of considerable interest that, in essence, the same structure is found in purple bacteria.
Cytochrome b6f
PS II and PS I are connected by a transmembrane proton pump, cytochrome b6f complex (plastoquinol—plastocyanin reductase; EC 1.10.99.1. Electrons from PS II are carried by plastoquinone to b6f, where they are removed in a stepwise fashion and transferred to a water-soluble electron carrier called plastocyanin. This redox process is coupled to the pumping of four protons across the membrane. The resulting proton gradient (together with the proton gradient produced by the water-splitting complex in PS II) is used to make ATP via ATP synthase.
The similarity in structure and function between cytochrome b6f (in chloroplasts) and cytochrome bc1 (Complex III in mitochondria) is striking. Both are transmembrane structures that remove electrons from a mobile, lipid-soluble electron carrier (plastoquinone in chloroplasts; ubiquinone in mitochondria) and transfer them to a mobile, water-soluble electron carrier (plastocyanin in chloroplasts; cytochrome c in mitochondria). Both are proton pumps that produce a transmembrane proton gradient.
Photosystem I
PS I accepts electrons from plastocyanin and transfers them either to NADPH (noncyclic electron transport) or back to cytochrome b6f (cyclic electron transport):
plastocyanin → P700 → P700* → FNR → NADPH ↑ ↓ b6f ← plastoquinonePS I, like PS II, is a complex, highly organized transmembrane structure that contains antenna chlorophylls, a reaction center (P700), phylloquinine, and a number of iron-sulfur proteins that serve as intermediate redox carriers.
The light-harvesting system of PS I uses multiple copies of the same transmembrane proteins used by PS II. The energy of absorbed light (in the form of delocalized, high-energy electrons) is funneled into the reaction center, where it excites special chlorophyll molecules (P700, maximum light absorption at 700 nm) to a higher energy level. The process occurs with astonishingly high efficiency.
Electrons are removed from excited chlorophyll molecules and transferred through a series of intermediate carriers to ferredoxin, a water-soluble electron carrier. As in PS II, this is a solid-state process that operates with 100% efficiency.
There are two different pathways of electron transport in PS I. In noncyclic electron transport, ferredoxin carries the electron to the enzyme ferredoxin NADP+ oxidoreductase that reduces NADP+ to NADPH. In cyclic electron transport, electrons from ferredoxin are transferred (via plastoquinone) to a proton pump, cytochrome b6f. They are then returned (via plastocyanin) to P700.
NADPH and ATP are used to synthesize organic molecules from CO2. The ratio of NADPH to ATP production can be adjusted by adjusting the balance between cyclic and noncyclic electron transport.
It is noteworthy that PS I closely resembles photosynthetic structures found in green sulfur bacteria, just as PS II resembles structures found in purple bacteria.
Photosynthetic electron transport chains in bacteria
PS II, PS I, and cytochromeb6f are found in chloroplasts. All plants and all photosynthetic algae contain chloroplasts, which produce NADPH and ATP by the mechanisms described above. In essence, the same transmembrane structures are also found in cyanobacteria.
Unlike plants and algae, cyanobacteria are prokaryotes. They do not contain chloroplasts. Rather, they bear a striking resemblance to chloroplasts themselves. This suggests that organisms resembling cyanobacteria were the evolutionary precursors of chloroplasts. One imagines primitive eukaryotic cells taking up cyanobacteria as intracellular symbionts.
Cyanobacteria
Cyanobacteria contain structures similar to PS II and PS I in chloroplasts. Their light-harvesting system is different from that found in plants (they use phycobilins, rather than chlorophylls, as antenna pigments), but their electron transport chain
H2O → PS II → plastoquinone → b6f → cytochrome c6 → PS I → ferredoxin → NADPH ↑ ↓ b6f ← plastoquinoneis, in essence, the same as the electron transport chain in chloroplasts. The mobile water-soluble electron carrier is cytochrome c6 in cyanobacteria, plastocyanin in plants.
Cyanobacteria can also synthesize ATP by oxidative phosphorylation, in the manner of other bacteria. The electron transport chain is
NADH dehydrogenase → plastoquinone → b6f → cytochrome c6 → cytochrome aa3 → O2where the mobile electron carriers are plastoquinone and cytochrome c6, while the proton pumps are NADH dehydrogenase, b6f and cytochrome aa3.
Cyanobacteria are the only bacteria that produce oxygen during photosynthesis. Earth's primordial atmosphere was anoxic. Organisms like cyanobacteria produced our present-day oxygen-containing atmosphere.
The other two major groups of photosynthetic bacteria, purple bacteria and green sulfur bacteria, contain only a single photosystem and do not produce oxygen.
Purple bacteria
Purple bacteria contain a single photosystem that is structurally related to PS II in cyanobacteria and chloroplasts:
- P870 → P870* → ubiquinone → bc1 → cytochrome c2 → P870
This is a cyclic process in which electrons are removed from an excited chlorophyll molecule (bacteriochlorophyll; P870), passed through an electron transport chain to a proton pump (cytochrome bc1 complex, similar but not identical to cytochrome bc1 in chloroplasts), and then returned to the cholorophyll molecule. The result is a proton gradient, which is used to make ATP via ATP synthase. As in cyanobacteria and chloroplasts, this is a solid-state process that depends on the precise orientation of various functional groups within a complex transmembrane macromolecular structure.
To make NADPH, purple bacteria use an external electron donor (hydrogen, hydrogen sulfide, sulfur, sulfite, or organic molecules such as succinate and lactate) to feed electrons into a reverse electron transport chain.
Green sulfur bacteria
Green sulfur bacteria contain a photosystem that is analogous to PS I in chloroplasts:
P840 → P840* → ferredoxin → NADH ↑ ↓ cyt c553 ← bc1 ← menaquinone
There are two pathways of electron transfer. In cyclic electron transfer, electrons are removed from an excited chlorophyll molecule, passed through an electron transport chain to a proton pump, and then returned to the chlorophyll. The mobile electron carriers are, as usual, a lipid-soluble quinone and a water-soluble cytochrome. The resulting proton gradient is used to make ATP.In noncyclic electron transfer, electrons are removed from an excited chlorophyll molecule and used to reduce NAD+ to NADH. The electrons removed from P840 must be replaced. This is accomplished by removing electrons from H2S, which is oxidized to sulfur (hence the name "green sulfur bacteria").
Purple bacteria and green sulfur bacteria occupy relatively minor ecological niches in the present day biosphere. They are of interest because of their importance in precambrian ecologies, and because their methods of photosynthesis were the likely evolutionary precursors of those in modern plants.
History
The first ideas about light's being used in photosynthesis were proposed by Colin Flannery in 1779[3] who recognized it was sunlight falling on plants that was required, although Joseph Priestly had noted the production of oxygen without the association with light in 1772.[4] Cornelius Van Niel proposed in 1931 that photosynthesis is a case of general mechanism where a photon of light is used to photo decompose a hydrogen donor and the hydrogen being used to reduce CO2.[5] Then in 1939 Robin Hill showed that isolated chloroplasts would make oxygen, but not fix CO2 showing the light and dark reactions occurred in different places[6]. This led later to the discovery of photosystem 1 and 2.
See also
References
- ^ Rajni Govindjee. "The Z-Scheme Diagram of Photosynthesis". http://www.life.uiuc.edu/govindjee/ZSchemeG.html. Retrieved March 2, 2006.
- ^ "Photosynthesis". McGraw Hill Encyclopedia of Science and Technology. 2007. p. 472.
- ^ Ingenhousz, J (1779). Experiments Upon Vegetables. London: Elmsly and Payne. http://books.google.com/books?id=7II5AAAAcAAJ&pg=PR1.
- ^ Priestley, J (1772). Observations on Different Kinds of Air. 62. London: Phil. Trans. Roy. Soc.. pp. 147–264. doi:10.1098/rstl.1772.0021.
- ^ van Niel, C. B. (1931.). "On the morphology and physiology of the purple and green sulfur bacteria". Arch. Microbial 3: 1–114. doi:10.1007/BF00454965.
- ^ Hill, R. (May 1939). "Oxygen Produced by Isolated Chloroplasts". Proceedings of the Royal Society of London. Series B, Biological Sciences 127 (847): 192–210. doi:10.1098/rspb.1939.0017.
Categories:- Light reactions
Wikimedia Foundation. 2010.