- Cholinesterase
-
acetylcholinesterase (Yt blood group) 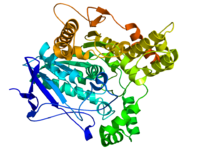
Diagram of Pacific electric ray acetylcholinesterase. From PDB 1EA5. Identifiers Symbol ACHE Alt. symbols YT Entrez 43 HUGO 108 OMIM 100740 RefSeq NM_015831 UniProt P22303 Other data EC number 3.1.1.7 Locus Chr. 7 q22 butyrylcholinesterase 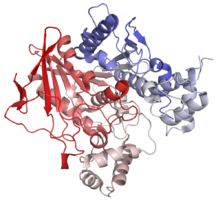
Cartoon diagram of human butyrylcholinesterase. From PDB 1P0I. Identifiers Symbol BCHE Alt. symbols CHE1 Entrez 590 HUGO 983 OMIM 177400 RefSeq NM_000055 UniProt P06276 Other data EC number 3.1.1.8 Locus Chr. 3 q26.1-26.2 In biochemistry, cholinesterase is a family of enzymes that catalyze the hydrolysis of the neurotransmitter acetylcholine into choline and acetic acid, a reaction necessary to allow a cholinergic neuron to return to its resting state after activation.
Contents
Types
There are two types:
- Acetylcholinesterase (EC 3.1.1.7) (AChE), also known as RBC cholinesterase, erythrocyte cholinesterase, or (most formally) acetylcholine acetylhydrolase, found primarily in the blood and neural synapses. Acetylcholinesterase exists in multiple molecular forms. In the mammalian brain the majority of AChE occurs as a tetrameric, G4 form (10) with much smaller amounts of a monomeric G1 (4S) form. [1]
- Pseudocholinesterase (EC 3.1.1.8) (BChE or BuChE), also known as plasma cholinesterase, butyrylcholinesterase, or (most formally) acylcholine acylhydrolase, found primarily in the liver.
The difference between the two types of cholinesterase has to do with their respective preferences for substrates: the former hydrolyses acetylcholine more quickly; the latter hydrolyses butyrylcholine more quickly.
The half-life of pseudocholinesterase is approximately 8–16 hours. Pseudocholinesterase levels may be reduced in patients with advanced liver disease. The decrease must be greater than 75% before significant prolongation of neuromuscular blockade occurs with succinylcholine.[2][3]
History
In 1968, Walo Leuzinger et al. successfully purified and crystallized the enzyme from electric eels at Columbia University, NY.[4][5]
The 3D structure of acetylcholinesterase was first determined in 1991 by Joel Sussman et al. using protein from the Pacific electric ray.[6]
Clinically-useful quantities of butyrylcholinesterase were synthesized in 2007 by PharmAthene, through the use of genetically-modified goats.[7]
Clinical significance
An absence or mutation of the pseudocholinesterase enzyme leads to a medical condition known as pseudocholinesterase deficiency. This is a silent condition that manifests itself only when people that have the deficiency receive the muscle relaxants succinylcholine or mivacurium during a surgery.
Pseudocholinesterase deficiency may also affect local anaesthetic selection in dental procedures. The enzyme plays an important role in the metabolism of ester-based local anaesthetics, a deficiency lowers the margin of safety and increases the risk of systemic effects with this type of anaesthetic. The selection of an amide-based solution is recommended in such patients.
Elevation of plasma pseudocholinesterase was observed in 90.5% cases of acute myocardial infarction.[8]
The presence of acetylcholinesterase in the amniotic fluid may be tested in early pregnancy. A sample of amniotic fluid is removed by amniocentesis, and presence of AChE can confirm several common types of birth defect, including abdominal wall defects and neural tube defects.[9]
Butyrylcholinesterase can be used as a prophylactic agent against nerve gas and other organophosphate poisoning.[7]
Cholinesterase inhibitors
A cholinesterase inhibitor (or "anticholinesterase") suppresses the action of the enzyme. Because of its essential function, chemicals that interfere with the action of cholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death (examples are some snake venoms, and the nerve gases sarin and VX). One counteracting medication is pralidoxime. The so-called nerve gases and many substances used in insecticides have been shown to act by combining with a residue of serine in the active site of acetylcholine esterase, inhibiting the enzyme completely. The enzyme acetylcholine esterase breaks down the neurotransmitter acetylcholine, which is released at nerve and muscle junctions in order to allow the muscle or organ to relax. The result of acetylcholine esterase inhibition is that acetylcholine builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop.
Among the most common acetylcholinesterase inhibitors are phosphorus-based compounds, which are designed to bind to the active site of the enzyme. The structural requirements are a phosphorus atom bearing two lipophilic groups, a leaving group (such as a halide or thiocyanate), and a terminal oxygen. The entry on Lawesson's reagent has some details on one sub-class of the phosphorus-based compounds.
Some benzodiazepines, e.g. temazepam have an inhibitory effect on cholinesterase.[10]
Outside of biochemical warfare, anticholinesterases are also used for reversing medication induced paralysis during anesthesia; as well as in the treatment of myasthenia gravis, glaucoma, and Alzheimer's disease. Such compounds are used for killing insects in a range of products including sheep dip, organophosphate pesticides, and carbamate pesticides. In addition to acute poisoning as described above, a semi-acute poisoning characterized by strong mental disturbances can occur. Also, prolonged exposure can cause birth defects.
Pop culture
- On season eight of Law and Order: SVU, Olivia Benson is taken to the hospital after being exposed to organophosphates, where she is told her cholinesterase level is low.
- In the film The I Inside, Simon Cable is poisoned with cholinesterase inhibitors and he is given atropine and pralidoxime to help reverse the poison. The doctor is also shown dispensing a diazepam at the beginning of the movie, which is contraindicated in cholinesterase inhibition.
- In the film The Rock, Dr. Goodspeed (Nicolas Cage) describes the effects of the chemical weapon VX gas as a cholinesterase inhibitor to John Mason (Sean Connery) as they remove guidance chips from the rocket designed to deliver the gas into San Francisco from Alcatraz.
- Indie band Ruet Caelum have a song entitled 'Showing Signs of Cholinesterase Inhabition"
Additional images
References
- ^ Wang R, Tang XC (2005). "Neuroprotective Effects of Huperzine A.". Neurosignals 14 (1-2): 71–82. doi:10.1159/000085387. PMID 15956816.
- ^ Brash: Clinical Anesthesia, 5th ed, pp 546-549
- ^ Miller: Anesthesia, 6th ed, pp 487-488
- ^ Leuzinger W, Baker AL (February 1967). "Acetylcholinesterase, I. Large-scale purification, homogeneity, and amino acid analysis". Proc. Natl. Acad. Sci. U.S.A. 57 (2): 446–451. doi:10.1073/pnas.57.2.446. PMC 335526. PMID 16591490. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=335526.
- ^ Leuzinger W, Baker AL, Cauvin E (February 1968). "Acetylcholinesterase. II. Crystallization, absorption spectra, isoionic point". Proc. Natl. Acad. Sci. U.S.A. 59 (2): 620–3. doi:10.1073/pnas.59.2.620. PMC 224717. PMID 5238989. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=224717.
- ^ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I (August 1991). "Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein". Science 253 (5022): 872–9. doi:10.1126/science.1678899. PMID 1678899.
- ^ a b Huang YJ, Huang Y, Baldassarre H, Wang B, Lazaris A, Leduc M, Bilodeau AS, Bellemare A, Côté M, Herskovits P, Touati M, Turcotte C, Valeanu L, Lemée N, Wilgus H, Bégin I, Bhatia B, Rao K, Neveu N, Brochu E, Pierson J, Hockley DK, Cerasoli DM, Lenz DE, Karatzas CN, Langermann S (August 2007). "Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning". Proc. Natl. Acad. Sci. U.S.A. 104 (34): 13603–8. doi:10.1073/pnas.0702756104. PMC 1934339. PMID 17660298. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1934339. Lay summary – BBC News.
- ^ Textbook of Medical Biochemistry, MN Chatterjea & Rana Shinde, 6th Ed, 2005 (Pg 565)
- ^ FBR Resource Guide: Acetylcholinesterase-Amniotic Fluid. Foundation for Blood Research (September 7, 2007). Retrieved on 2007-11-21.
- ^ Holmes JH; Kanfer I, Zwarenstein H. (August 1978). "Effect of benzodiazepine derivatives on human blood cholinesterase in vitro.". Res Commun Chem Pathol Pharmacol 21 (2): 367–70. PMID 29327.
External links
- ATSDR Case Studies in Environmental Medicine: Cholinesterase Inhibitors, Including Insecticides and Chemical Warfare Nerve Agents U.S. Department of Health and Human Services
- Movies at weizmann.ac.il showing the structure of acetylcholinesterase and interactions with various inhibitors.
- Proteopedia Acetylcholinesterase
- PDB Molecule of the Month pdb54_1
- MeSH Acetylcholinesterase
- MeSH Pseudocholinesterase
monoamine glutamate→GABAanabolism: Glutamate decarboxylase
catabolism: 4-aminobutyrate aminotransferase · 4-aminobutyrate transaminasearginine→NO choline→Acetylcholine anabolism: Choline acetyltransferase
catabolism: Cholinesterase (Acetylcholinesterase, Butyrylcholinesterase)Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Genes on chromosome 7
- Genes on chromosome 3
- Acetylcholine
- Peripheral membrane proteins
- EC 3.1.1
Wikimedia Foundation. 2010.
Look at other dictionaries:
cholinestérase — [ kɔlinɛsteraz ] n. f. • 1935; angl. cholinesterase (1932); de choline et estérase ♦ Biochim. Enzyme qui hydrolyse l acétylcholine en choline et acide acétique, et joue un rôle important dans le fonctionnement du système nerveux. ● cholinestérase … Encyclopédie Universelle
Cholinesterase — Cholinestérase L acétylcholinestérase est la principale enzyme du genre cholinestérase. En biochimie une cholinestérase est une enzyme qui catalyse la réaction d hydrolyse d un ester de la choline (acétylcholine, butyrylcholine) en c … Wikipédia en Français
cholinesterase — [kō΄lənes′tər ās΄] n. [< CHOLINE + ESTERASE] an enzyme which hydrolyzes a choline ester; esp., acetylcholine, which converts to choline and acetic acid, thus canceling a nerve impulse transmission … English World dictionary
Cholinesterase — Cholinesterasen (ChE) sind Enzyme, die Cholin Ester spalten. Sie sind im Stoffwechsel von vielzelligen Tieren unverzichtbar zum Abbau dieser Stoffe, insbesondere des Acetylcholins, einem Neurotransmitter. Es gibt zwei Cholinesterasen.… … Deutsch Wikipedia
Cholinestérase — L acétylcholinestérase est la principale enzyme du genre cholinestérase. En biochimie une cholinestérase est une enzyme qui catalyse la réaction d hydrolyse d un ester de la choline (acétylcholine, butyrylcholine) en choline et en acide acétiqu … Wikipédia en Français
Cholinesterase enzyme — protein Name = acetylcholinesterase (Yt blood group) caption = Diagram of Pacific electric ray acetylcholinesterase. From PDB|1EA5. width = 200 HGNCid = 108 Symbol = ACHE AltSymbols = YT EntrezGene = 43 OMIM = 100740 RefSeq = NM 015831 UniProt =… … Wikipedia
cholinesterase — noun Date: 1932 1. acetylcholinesterase 2. an enzyme that hydrolyzes choline esters and that is found especially in blood plasma called also pseudocholinesterase … New Collegiate Dictionary
cholinesterase — /koh leuh nes teuh rays , rayz , kol euh /, n. Biochem. an enzyme, found esp. in the heart, brain, and blood, that hydrolyzes acetylcholine to acetic acid and choline. [1930 35; CHOLINE + ESTERASE] * * * … Universalium
cholinesterase — noun An enzyme, in muscles, nerves etc, that catalyzes the hydrolysis of acetylcholine … Wiktionary
cholinesterase — One of a family of enzymes capable of catalyzing the hydrolysis of acylcholines and a few other compounds. In mammals, found in white matter of brain, liver, heart, pancreas, and serum. It is also found in cobra venom. SEE ALSO … Medical dictionary


