- Naringenin
-
Not to be confused with naringin.
Naringenin 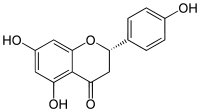
Systematic (IUPAC) name 5,7-dihydroxy-2-(4-hydroxyphenyl)chroman-4-one Clinical data Pregnancy cat. ? Legal status ? Identifiers CAS number 480-41-1 
ATC code None PubChem CID 932 DrugBank EXPT02295 UNII HN5425SBF2 
ChEMBL CHEMBL9352 
Chemical data Formula C15H12O5 Mol. mass 272.257 g/mol  (what is this?) (verify)
(what is this?) (verify)Naringenin is a flavanone, a type of flavonoid, that is considered to have a bioactive effect on human health as antioxidant, free radical scavenger, anti-inflammatory, carbohydrate metabolism promoter, and immune system modulator. It is the predominant flavanone in grapefruit.[1]
Biological effects
This substance has also been shown to reduce oxidative damage to DNA in vitro. Scientists exposed cells to 80 micromoles of naringenin per liter, for 24 hours, and found that the amount of hydroxyl damage to the DNA was reduced by 24% in that very short period of time.
Naringenin found in grapefruit juice has been shown to have an inhibitory effect on the human cytochrome P450 isoform CYP1A2, which can change pharmacokinetics in a human (or orthologous) host of several popular drugs in an adverse manner, even resulting in carcinogens of otherwise harmless substances.[2]
Naringenin has also been shown to reduce hepatitis C virus production by infected hepatocytes (liver cells) in cell culture. This seem to be secondary to Naringenin ability to inhibit the secretion of very-low-density lipoprotein by the cells.[3] The antiviral effects of naringenin are currently under clinical investigation.[4]
Naringenin seems to protect LDLR-deficient mice from the obesity effects of a high-fat diet.[5]
Naringenin lowers the plasma and hepatic cholesterol concentrations by suppressing HMG-CoA reductase and ACAT in rats fed a high-cholesterol diet.[6]
Sources and bioavailability
Grapefruit, Oranges, and Tomato (skin).
This bioflavonoid is difficult to absorb on oral ingestion. In the best-case scenario, only 15% of ingested naringenin will get absorbed in the human gastrointestinal tract.[citation needed] A full glass of orange juice will supply about enough naringenin to achieve a [blood plasma?] concentration of about 0.5 micromoles per liter.[citation needed]
The naringenin-7-glucoside form seems less bioavailable than the aglycol form.[7]
Grapefruit juice can provide much higher plasma concentrations of naringenin than orange juice.[8] Also found in grapefruit is the related compound Kaempferol, which has a hydroxyl group next to the ketone group.
Naringenin can be absorbed from cooked tomato paste.[9]
References
- ^ http://ajpgi.physiology.org/cgi/content/abstract/279/6/G1148 "Bioavailability of the flavanone naringenin and its glycosides in rats"
- ^ Edwards DJ, Bernier SM (1996). "Inhibitory effect of grapefruit juice and its bitter principal, naringenin, on CYP1A2 dependent metabolism of caffeine in man". Life Sciences 59 (13): 1025–1030. doi:10.1016/0024-3205(96)00417-1. PMC 1381556. PMID 8485024. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1381556.
- ^ Nahmias Y, Goldwasser J, Casali M, et al. (May 2008). "Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin". Hepatology 47 (5): 1437–45. doi:10.1002/hep.22197. PMID 18393287.
- ^ A Pilot Study of the Grapefruit Flavonoid Naringenin for HCV Infection
- ^ http://diabetes.diabetesjournals.org/content/early/2009/07/09/db09-0634.abstract "Naringenin prevents dyslipidemia, apoB overproduction and hyperinsulinemia in LDL-receptor null mice with diet-induced insulin resistance"
- ^ http://content.karger.com/ProdukteDB/produkte.asp?Aktion=ShowFulltext&ArtikelNr=12783&Ausgabe=224614&ProduktNr=223977 Annals of Nutrition & Metabolism 1999;43:173-180 (DOI: 10.1159/000012783) "Cholesterol-Lowering Activity of Naringenin via Inhibition of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase and Acyl Coenzyme A:Cholesterol Acyltransferase in Rats"
- ^ http://dx.doi.org/10.1006/bbrc.1999.1695 "doi:10.1006/bbrc.1999.1695"
- ^ http://jn.nutrition.org/cgi/content/abstract/131/2/235?ijkey=6cd7e72885072c7f69948835b6c36cbef4ab0e35&keytype2=tf_ipsecsha "Plasma Kinetics and Urinary Excretion of the Flavanones Naringenin and Hesperetin in Humans after Ingestion of Orange Juice and Grapefruit Juice"
- ^ http://jn.nutrition.org/cgi/content/full/132/11/3349 "Naringenin from Cooked Tomato Paste Is Bioavailable in Men"
Flavanones Alpinetin | Butin | Eriodictyol | Naringenin | PinocembrinO-methylated flavanones C-methylated flavanones PoriolGlycosides Eriocitrin | Hesperidin | Liquiritin | Naringin | Narirutin | Poncirin | SakuraninAcetylated 8-prenylnaringeninAcetylated glycosides NirurinCategories:- Flavanones
- Natural phenol drugs
- Resorcinols
Wikimedia Foundation. 2010.
