- Ditran
-
Ditran 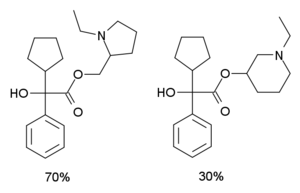
Systematic (IUPAC) name mixture of (1-ethylpyrrolidin-2-yl)methyl 2-cyclopentyl-2-hydroxy-2-phenylacetate and (1-ethylpiperidin-3-yl)-2-cyclopentyl-2-hydroxy-2-phenylacetate Clinical data Pregnancy cat. ? Legal status ? Identifiers CAS number 8015-54-1 
ATC code ? PubChem CID 111040 ChEMBL CHEMBL126644 
Chemical data Formula C20H29NO3 Mol. mass 331.448 g/mol  (what is this?) (verify)
(what is this?) (verify)Ditran (JB-329) is an anticholinergic drug mixture, related to the chemical warfare agent 3-Quinuclidinyl benzilate (QNB).
Ditran is composed of a mixture of 70% 1-ethyl- 2-pyrrolidinylmethyl- alpha- phenylcyclopentylglycolate and 30% 1-ethyl- 3-piperidyl- alpha- phenylcyclopentylglycolate. These compounds are structural isomers and have very similar pharmacological properties. The piperidine compound is the more potent of the two and the reason the mixture was used was because of ease of manufacture, however it is also possible to make the piperidine compound in its pure form, so there were ultimately two forms of Ditran used in research, the original 70/30 mix, and "Ditran-B", the pure piperidine compound. Ditran was developed during chemical weapons research in an attempt to produce non-lethal incapacitating agents, similar to QNB itself. The ditran mixture is more potent as an anticholinergic than the piperidyl benzilate drugs such as N- methyl- 3 -piperidyl benzilate, but is less potent than QNB.[1][2]
There has been a modest amount of scientific research using this mixture,[3][4] but most modern research using these kind of anticholinergic drugs uses N- methyl- 3- piperidyl benzilate due to its wider availability.
See also
References
- ^ Siegfried Franke, Peter Franz and Werner Warnke. Lehrbuch der Militärchemie Bd 1, Militärverlag der Deutschen Demokratischen Republik Ost-Berlin, Deutscher Militärverlag, 1974.
- ^ Possible Long-Term Health Effects of Short-Term Exposure to Chemical Agents, Volume 1 (1982). Commission on Life Sciences. The National Academies Press. pp191-195.
- ^ Volle, RL; Alkadhi, KA; Branisteanu, DD; Reynolds, LS; Epstein, PM; Smilowitz, H; Lambert, JJ; Henderson, EG (1982). "Ketamine and ditran block end-plate ion conductance and 3Hphencyclidine binding to electric organ membrane". The Journal of pharmacology and experimental therapeutics 221 (3): 570–6. PMID 6123584.
- ^ Vanderwolf, CH; Stewart, DJ (1986). "Joint cholinergic-serotonergic control of neocortical and hippocampal electrical activity in relation to behavior: effects of scopolamine, ditran, trifluoperazine and amphetamine". Physiology & behavior 38 (1): 57–65. doi:10.1016/0031-9384(86)90132-0. PMID 3786502.
Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Deliriants
- Anticholinergics
- Pyrrolidines
- Piperidines
- Carboxylate esters
- Alcohols
- Hallucinogen stubs
Wikimedia Foundation. 2010.
Look at other dictionaries:
DITRAN — DIagnostic Fortran ( > IEEE Standard Dictionary ) … Acronyms
DITRAN — DIagnostic Fortran ( > IEEE Standard Dictionary ) … Acronyms von A bis Z
N-Methyl-3-piperidyl benzilate — Systematic (IUPAC) name (1 methylpiperidin 3 yl) 2 hydroxy 2,2 di(phenyl)acetate Clinical data Pregnancy cat … Wikipedia
N-Ethyl-3-piperidyl benzilate — Systematic (IUPAC) name (1 ethylpiperidin 3 yl) 2 hydroxy 2,2 di(phenyl)acetate Clinical data Pregnancy cat … Wikipedia
CAR-302,196 — Systematic (IUPAC) name (1 methylpiperidin 4 yl) 2 cyclopentyl 2 hydroxypent 3 ynoate Clinical data Pregnancy cat … Wikipedia
Datura — This article is about the plant genus. For the Italian dance group, see Datura (band). For the former town in California, see Datura, California. For The Tori Amos song, see Datura (song). Datura Datura stramonium … Wikipedia
Mandrake (plant) — Mandrake root redirects here. For the Deep Purple song, see Mandrake Root. Mandragora redirects here. For other uses, see Mandragora (disambiguation). Mandrake Scientific classification Kingdom … Wikipedia
Phencyclidine — Systematic (IUPAC) name … Wikipedia
Solanaceae — Nightshade redirects here. For other uses, see Nightshade (disambiguation). Solanaceae A flowering Brugmansia suaveolens from the US Botanic Garden Scientific classification … Wikipedia
Scopolamine — Systematic (IUPAC) name (–) (S) 3 hydroxy 2 pheny … Wikipedia
