- Phosgene oxime
-
Phosgene oxime 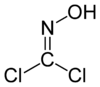
 dichloroformaldoximeOther namesdichloroformoxime, CX
dichloroformaldoximeOther namesdichloroformoxime, CXIdentifiers CAS number 1794-86-1 PubChem 65582 ChemSpider 59024 
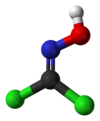
Jmol-3D images Image 1 - Cl/C(Cl)=N\O
Properties Molar mass 113.93 g mol−1 Appearance colorless crystalline solid or yellowish-brown liquid[1] Melting point 35-40 °C[1]
Boiling point 128 °C[1]
Solubility in water 70%[1] Hazards Main hazards highly toxic  oxime (verify) (what is:
oxime (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Phosgene oxime, or CX, is an organic compound with the formula Cl2CNOH. It is a potent chemical weapon, specifically a nettle agent. The compound itself is a colorless solid, but impure samples are often yellowish liquids. It has a strong, disagreeable odor and a violently irritating vapor.
Contents
Preparation and reactions
Phosgene oxime is generally prepared by reduction of chloropicrin:
- Cl3CNO2 + 2 Sn + 5 HCl + H2O → Cl2C=N-OH + 2 H3O[SnCl3]
The observation of a transient violet color in the reaction suggests intermediate formation of trichloronitrosomethane (Cl3CNO). Early preparations, using stannous chloride as the reductant, also started with chloropicrin[2]
The compound is electrophilic and thus sensitive to nucleophiles, including base hydrolysis:
- Cl2CNOH + 2 NaOH → CO2 + NH2OH + 2 NaCl + H2O
Similar processes provide an easy way to destroy this dangerous compound. Hydrazine converts it to HCN and N2.
Safety
Phosgene oxime is a vesicant.[3] It is toxic by inhalation, ingestion, or skin contact. The effects of the poisoning occur almost immediately. No antidote for phosgene oxime poisoning is known. Generally, any treatment is supportive. Typical physical symptoms of CX exposure are as follows:
- Skin: Blanching surrounded by an erythematous ring can be observed within 30 seconds of exposure. A wheal develops on exposed skin within 30 minutes. The original blanched area acquires a brown pigmentation by 24 hours. An eschar forms in the pigmented area by 1 week and sloughs after approximately 3 weeks. Initially, the effects of CX can easily be misidentified as mustard gas exposure. However, the onset of skin irritation resulting from CX exposure is a great deal faster than mustard gas, which typically takes several hours or more to cause skin irritation.
- Eyes: Eye examination typically demonstrates conjunctivitis, lacrimation, lid edema, and blepharospasm after even minute exposures. More severe exposures can result in keratitis, iritis, corneal perforation, and blindness.
- Respiratory: Irritation of the mucous membranes may be observed on examination of the oropharynx and nose. Evidence of pulmonary edema, including rales and wheezes, may be noted on auscultation. Pulmonary thromboses are prominent features of severe CX exposure.
- Gastrointestinal: Some animal data suggest that CX may cause hemorrhagic inflammatory changes in the GI tract.
Decontamination, treatment, and handling properties
Phosgene oxime is highly soluble in water. It is corrosive to metals and also decomposes on contact with metals. It is rapidly hydrolysed by alkaline solutions. Adsorbent powders such as fullers earth or talcum powder can also be used.
References
- ^ a b c d ATSDR Medical Management Guidelines for Phosgene Oxime
- ^ Prandtl, Wilhelm; Dollfus, Werner "Über das Trichlor-nitroso-methan, das Dichlor-formoxim (Phosgen-oxim) und einige ihrer Derivate, 2. Mitteil.: Über zwei neue Derivate der Kohlensäure" Berichte der Deutschen Chemischen Gesellschaft, 1932, volume 65B, 754-9. doi:10.1002/cber.19320650515
- ^ McManus John; Huebner Kermit "Vesicants" Critical care clinics (2005), 21(4), 707-18. doi:10.1016/j.ccc.2005.06.005
External links
- EMedicine: Urticants, Phosgene Oxime
- Center for the Study of Bioterrorism: Phosgene Oxime
- Centers for Disease Control: Facts About Phosgene Oxime
- Virtual Naval Hospital: Phosgene Oxime
Agents used in chemical warfare Blood Blister Ethyldichloroarsine (ED) · Methyldichloroarsine (MD) · Phenyldichloroarsine (PD) · Lewisite (L) · Sulfur mustard (HD · H · HT · HL · HQ) · Nitrogen mustard (HN1 · HN2 · HN3)
Nerve Pulmonary Incapacitating Riot control Categories:- Chemical weapons
- Organochlorides
- Oximes
Wikimedia Foundation. 2010.
