- Atropine
-
Atropine 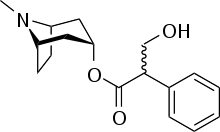
Systematic (IUPAC) name (RS)-(8-methyl-8-azabicyclo[3.2.1]oct-3-yl) 3-hydroxy-2-phenylpropanoate Clinical data Trade names Atropen AHFS/Drugs.com monograph MedlinePlus a682487 Pregnancy cat. C(US) Legal status Rx only Routes Oral, IV, IM, rectal Pharmacokinetic data Bioavailability 25% Metabolism 50% hydrolysed to tropine and tropic acid Half-life 2 hours Excretion 50% excreted unchanged in urine Identifiers CAS number 51-55-8 
ATC code A03BA01 S01FA01 PubChem CID 174174 IUPHAR ligand 320 DrugBank DB00572 ChemSpider 10194105 
UNII 7C0697DR9I 
KEGG D00113 
ChEBI CHEBI:16684 
ChEMBL CHEMBL195 
Chemical data Formula C17H23NO3 Mol. mass 289.369 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Atropine is a naturally occurring tropane alkaloid extracted from deadly nightshade (Atropa belladonna), Jimson weed (Datura stramonium), mandrake (Mandragora officinarum) and other plants of the family Solanaceae. It is a secondary metabolite of these plants and serves as a drug with a wide variety of effects. It is a competitive antagonist for the muscarinic acetylcholine receptor. It is classified as an anticholinergic drug (parasympatholytic). The species name "belladonna" comes from the original use of deadly nightshade as a way of dilating women's pupils to make them beautiful. Both atropine and the genus name for deadly nightshade derive from Atropos, one of the three Fates who, according to Greek mythology, chose how a person was to die. Atropine is a core medicine in the World Health Organization's "Essential Drugs List", which is a list of minimum medical needs for a basic health care system.[1]
Contents
Physiological effects and uses
Atropine increases firing of the sinoatrial node (SA) and conduction through the atrioventricular node (AV) of the heart, opposes the actions of the vagus nerve, blocks acetylcholine receptor sites, and decreases bronchial secretions.
In general, atropine lowers the parasympathetic activity of all muscles and glands regulated by the parasympathetic nervous system. This occurs because atropine is a competitive antagonist of the muscarinic acetylcholine receptors (acetylcholine being the main neurotransmitter used by the parasympathetic nervous system). Therefore, it may cause swallowing difficulties and reduced secretions.
Ophthalmic use
Topical atropine is used as a cycloplegic, to temporarily paralyze the accommodation reflex, and as a mydriatic, to dilate the pupils. Atropine degrades slowly, typically wearing off in 7 to 14 days, so it is generally used as a therapeutic mydriatic, whereas tropicamide (a shorter-acting cholinergic antagonist) or phenylephrine (an α-adrenergic agonist) is preferred as an aid to ophthalmic examination. Atropine induces mydriasis by blocking contraction of the circular pupillary sphincter muscle, which is normally stimulated by acetylcholine release, thereby allowing the radial pupillary dilator muscle to contract and dilate the pupil. Atropine induces cycloplegia by paralyzing the ciliary muscles, whose action inhibits accommodation to allow accurate refraction in children, helps to relieve pain associated with iridocyclitis, and treats ciliary block (malignant) glaucoma. Atropine is contraindicated in patients pre-disposed to narrow angle glaucoma.
Atropine can be given to patients who have direct globe trauma.
Resuscitation
Injections of atropine are used in the treatment of bradycardia (an extremely low heart rate). Atropine blocks the action of the vagus nerve, a part of the parasympathetic system of the heart whose main action is to decrease heart rate. Therefore, its primary function in this circumstance is to increase the heart rate. Atropine was previously included in international resuscitation guidelines for use in cardiac arrest associated with asystole and PEA, but was removed from these guidelines in 2010 due to a lack of evidence.[2] For symptomatic bradycardia, the usual dosage is 0.5 to 1 mg IV push, may repeat every 3 to 5 minutes up to a maximum dose of 3 mg.[3]
Atropine is also useful in treating second-degree heart block Mobitz Type 1 (Wenckebach block), and also third-degree heart block with a high Purkinje or AV-nodal escape rhythm. It is usually not effective in second-degree heart block Mobitz type 2, and in third-degree heart block with a low Purkinje or ventricular escape rhythm. Atropine is contraindicated in ischemia-induced conduction block, because the drug increases oxygen demand of the AV nodal tissue, thereby aggravating ischemia and the resulting heart block.
One of the main actions of the parasympathetic nervous system is to stimulate the M2 muscarinic receptor in the heart, but atropine inhibits this action.
Secretions and bronchoconstriction
Atropine's actions on the parasympathetic nervous system inhibits salivary and mucus glands. The drug may also inhibit sweating via the sympathetic nervous system. This can be useful in treating hyperhidrosis, and can prevent the death rattle of dying patients. Even though atropine has not been officially indicated for either of these purposes by the FDA, it has been used by physicians for these purposes.[4]
Treatment for organophosphate poisoning
Atropine is not an actual antidote for organophosphate poisoning. However, by blocking the action of acetylcholine at muscarinic receptors, atropine also serves as a treatment for poisoning by organophosphate insecticides and nerve gases, such as tabun (GA), sarin (GB), soman (GD) and VX. Troops who are likely to be attacked with chemical weapons often carry autoinjectors with atropine and obidoxime, which can be quickly injected into the thigh. Atropine is often used in conjunction with pralidoxime chloride.
Atropine is given as a treatment for SLUDGE (salivation, lacrimation, urination, diaphoresis, gastrointestinal motility, emesis) symptoms caused by organophosphate poisoning. Another mnemonic is DUMBBELSS, which stands for diarrhea, urination, miosis, bradycardia, bronchoconstriction, excitation (as of muscle in the form of fasciculations and CNS), lacrimation, salivation, and sweating (only sympathetic innervation using Musc receptors).
Some of the nerve agents attack and destroy acetylcholinesterase by phosphorylation, so the action of acetylcholine becomes prolonged, pralidoxime (2-PAM) is the cure for organophosphate poisoning because it can cleave this phosphorylation. Atropine can be used to reduce the effect of the poisoning by blocking muscarinic acetylcholine receptors, which would otherwise be overstimulated by excessive acetylcholine accumulation.
Optical penalisation
In refractive and accommodative amblyopia, when occlusion is not appropriate sometimes atropine is given to induce blur in the good eye.[5]
Side-effects and overdose
Adverse reactions to atropine include ventricular fibrillation, supraventricular or ventricular tachycardia, dizziness, nausea, blurred vision, loss of balance, dilated pupils, photophobia, dry mouth and potentially extreme confusion, dissociative hallucinations and excitation especially amongst the elderly. These latter effects are because atropine is able to cross the blood-brain barrier. Because of the hallucinogenic properties, some have used the drug recreationally, though this is potentially dangerous and often unpleasant.
In overdoses, atropine is poisonous. Atropine is sometimes added to potentially addictive drugs, particularly anti-diarrhea opioid drugs such as diphenoxylate or difenoxin, wherein the secretion-reducing effects of the atropine can also aid the anti-diarrhea effects.
Although atropine treats bradycardia (slow heart rate) in emergency settings, it can cause paradoxical heart rate slowing when given at very low doses, presumably as a result of central action in the CNS.[6]
Atropine is incapacitating at doses of 10 to 20 mg per person. Its LD50 is estimated to be 453 mg per person (per oral) with a probit slope of 1.8.[7] The antidote to atropine is physostigmine or pilocarpine.
A common mnemonic used to describe the physiologic manifestations of atropine overdose is: as per Jon Blinkey "hot as a hare, blind as a bat, dry as a bone, red as a beet, and mad as a hatter".[8] These associations reflect the specific changes of warm, dry skin from decreased sweating, blurry vision, decreased sweating/lacrimation, vasodilation, and central nervous system effects on muscarinic receptors, type 4 and 5. This set of symptoms is known as anticholinergic toxidrome, and may also be caused by other drugs with anticholinergic effects, such as diphenhydramine, phenothiazine antipsychotics and benztropine.[9]
Chemistry and pharmacology
Atropine is a racemic mixture of d-hyoscyamine and l-hyoscyamine, with most of its physiological effects due to l-hyoscyamine. Its pharmacological effects are due to binding to muscarinic acetylcholine receptors. It is an antimuscarinic agent. Significant levels are achieved in the CNS within 30 minutes to 1 hour and disappears rapidly from the blood with a half-life of 2 hours. About 60% is excreted unchanged in the urine, most of the rest appears in urine as hydrolysis and conjugation products. Effects on the iris and ciliary muscle may persist for longer than 72 hours.
The most common atropine compound used in medicine is atropine sulfate (C17H23NO3)2·H2SO4·H2O, the full chemical name is 1α H, 5α H-Tropan-3-α ol (±)-tropate(ester), sulfate monohydrate.
The vagus (parasympathetic) nerves that innervate the heart release acetylcholine (ACh) as their primary neurotransmitter. ACh binds to muscarinic receptors (M2) that are found principally on cells comprising the sinoatrial (SA) and atrioventricular (AV) nodes. Muscarinic receptors are coupled to the Gi-protein; therefore, vagal activation decreases cAMP. Gi-protein activation also leads to the activation of KACh channels that increase potassium efflux and hyperpolarizes the cells.
Increases in vagal activity to the SA node decreases the firing rate of the pacemaker cells by decreasing the slope of the pacemaker potential (phase 4 of the action potential); this decreases heart rate (negative chronotropy). The change in phase 4 slope results from alterations in potassium and calcium currents, as well as the slow-inward sodium current that is thought to be responsible for the pacemaker current (If). By hyperpolarizing the cells, vagal activation increases the cell's threshold for firing, which contributes to the reduction the firing rate. Similar electrophysiological effects also occur at the AV node; however, in this tissue, these changes are manifested as a reduction in impulse conduction velocity through the AV node (negative dromotropy). In the resting state, there is a large degree of vagal tone on the heart, which is responsible for low resting heart rates.
There is also some vagal innervation of the atrial muscle, and to a much lesser extent, the ventricular muscle. Vagus activation, therefore, results in modest reductions in atrial contractility (inotropy) and even smaller decreases in ventricular contractility.
Muscarinic receptor antagonists bind to muscarinic receptors thereby preventing ACh from binding to and activating the receptor. By blocking the actions of ACh, muscarinic receptor antagonists very effectively block the effects of vagal nerve activity on the heart. By doing so, they increase heart rate and conduction velocity.
History
Mandragora (mandrake) was described by Theophrastus in the fourth century B.C. for treatment of wounds, gout, and sleeplessness, and as a love potion. By the first century A.D. Dioscorides recognized wine of mandrake as an anaesthetic for treatment of pain or sleeplessness, to be given prior to surgery or cautery.[8] The use of Solanaceae containing tropane alkaloids for anesthesia, often in combination with opium, persisted throughout the Roman and Islamic Empires and continued in Europe until superseded by the use of ether, chloroform, and other modern anesthetics.
Atropine extracts from the Egyptian henbane were used by Cleopatra in the last century B.C. to dilate her pupils, in the hope that she would appear more alluring. In the Renaissance, women used the juice of the berries of Atropa belladonna to enlarge the pupils of their eyes, for cosmetic reasons; "bella donna" is Italian for "beautiful lady". This practice resumed briefly in the late nineteenth- and early twentieth-century in Paris.
The mydriatic effects of atropine were studied among others by the German chemist Friedlieb Ferdinand Runge (1795–1867). In 1831, the pharmacist Mein succeeded the pure crystalline isolation of atropine. The substance was first synthesized by German chemist Richard Willstätter in 1901.
Natural sources
Atropine is found in many members of the Solanaceae family. The most commonly-found sources are Atropa belladonna, Datura inoxia, D. metel, and D. stramonium. Other sources include members of the Brugmansia and Hyoscyamus genera. The Nicotiana genus (including the tobacco plant, N. tabacum) is also found in the Solanaceae family, but these plants do not contain atropine or other tropane alkaloids.
Synthesis
Atropine can be synthesized by the reaction of tropine with tropic acid in the presence of hydrochloric acid.
See also
References
- ^ "WHO Model List of Essential Medicines" (PDF). World Health Organization. March 2005. http://whqlibdoc.who.int/hq/2005/a87017_eng.pdf. Retrieved 2006-03-12.
- ^ Field JM, Hazinski MF, Sayre MR, et al. (November 2010). "Part 1: executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation 122 (18 Suppl 3): S640–56. doi:10.1161/CIRCULATIONAHA.110.970889. PMID 20956217. http://circ.ahajournals.org/cgi/content/full/122/18_suppl_3/S640.
- ^ * Bryan E, Bledsoe; Robert S. Porter, Richard A. Cherry (2004). "Ch. 3". Intermediate Emergency Care. Upper Saddle River, NJ: Pearson Prentice Hill. pp. 260. ISBN 0-13-113607-0.
- ^ Death Rattle and Oral Secretions
- ^ Georgievski Z, Koklanis K, Leone J. Fixation behaviour in the treatment of amblyopia using atropine. Clinical and Experimental Ophthalmology 2008; 36 (Suppl 2): A764–A765.
- ^ * Rang HP, Dale MM, Ritter JM, Flower RJ (2007). "Ch. 10". Rang and Dale's Pharmacology. Elsevier Churchill Livingstone. pp. 153. ISBN 0-443-06911-5.
- ^ * Goodman E (2010). Ketchum J, Kirby R. ed. Historical Contributions to the Human Toxicology of Atropine. Eximdyne. pp. 120. ISBN 978-0-9677264-3-4.
- ^ a b Robert S. Holzman, MD (1998-07). "The Legacy of Atropos". Anesthesiology 89 (1): 241–249. PMID 9667313. http://www.anesthesiology.org/pt/re/anes/fulltext.00000542-199807000-00030.htm;jsessionid=GSJKLv9vLCdQSmpp6vH3xdhnzWN1hy3s7JqMNFpWkHhLbKJT5vLM!741375937!-949856145!8091!-1#P89. Retrieved 2007-05-21. citing J. Arena, Poisoning: Toxicology-Symptoms-Treatments, 3rd edition. Springfield, Charles C. Thomas, 1974, p 345
- ^ Szajewski J (1995). "Acute anticholinergic syndrome". IPCS Intox Databank. http://www.intox.org/databank/documents/treat/treate/trt05_e.htm. Retrieved 2007-05-22.
External links
Drugs for functional gastrointestinal disorders (A03) Drugs for
functional bowel disordersTertiary
amino groupQuaternary ammonium
compoundsBenzilone • Mepenzolate • Pipenzolate • Glycopyrronium • Oxyphenonium • Penthienate • Methantheline • Propantheline • Otilonium bromide • Tridihexethyl • Isopropamide • Hexocyclium • Poldine • Bevonium • Diphemanil • Tiemonium iodide • Prifinium bromide • Timepidium bromide • FenpiveriniumActing on serotonin receptorsOtherFenpiprane • Diisopromine • Chlorbenzoxamine • Pinaverium • Fenoverine • Idanpramine • Proxazole • Alverine • Trepibutone • Isometheptene • Caroverine • Phloroglucinol • Silicones • TrimethyldiphenylpropylamineBelladonna and derivatives
(antimuscarinics)tertiary amines: Atropine • Hyoscyamine
quaternary ammonium compounds: Scopolamine (Butylscopolamine, Methylscopolamine) • Methylatropine • Fentonium • Cimetropium bromidePropulsives Ophthalmologicals: mydriatics and cycloplegics (S01F) Anticholinergics/antimuscarinics Sympathomimetics M: EYE
anat(g/a/p)/phys/devp/prot
noco/cong/tumr, epon
proc, drug(S1A/1E/1F/1L)
Ophthalmologicals: antiglaucoma preparations and miotics (S01E) Sympathomimetics Parasympathomimetics muscarinicmuscarinic/nicotinicCarbonic anhydrase inhibitors/
(sulfonamides)Beta blocking agents Prostaglandin analogues (F2α) Other agents M: EYE
anat(g/a/p)/phys/devp/prot
noco/cong/tumr, epon
proc, drug(S1A/1E/1F/1L)
Ancient anaesthesia Plants/animals Aconite • Castoreum • Cannabis • Coca • Deadly nightshade • Henbane • Lactucarium • Mandrake • Metel nut • Opium • Poison hemlock • Saussurea • Toloatzin • WillowPeople Abulcasis • Avenzoar • Avicenna • Celsus • Dioscorides • Galen • Hippocrates • Rhazes • Sabuncuoğlu • Sushrutha • Theophrastus • ZhangCompounds Aconitine • Δ9-THC • Atropine • Cocaine • Coniine • Hyoscyamine • Morphine • Salicylate • ScopolamineHealth science > Medicine > Emergency medicine Procedures Acute Care of at-Risk Newborns (ACoRN) · Advanced cardiac life support (ACLS) · Advanced Trauma Life Support (ATLS) · Cardiopulmonary resuscitation (CPR) · First aid · Neonatal Resuscitation Program (NRP) · Pediatric Advanced Life Support (PALS) · Basic Life SupportEquipment Bag valve mask (BVM) · Chest tube · Defibrillation (AED, ICD) · Electrocardiogram (ECG/EKG) · Intraosseous infusion (IO) · Intravenous therapy (IV) · Tracheal intubation · Nasopharyngeal airway (NPA) · Oropharyngeal airway (OPA) · Pocket maskDrugs Other Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Natural tropane alkaloids
- Antidotes
- Deliriants
- Entheogens
- Muscarinic antagonists
- World Health Organization essential medicines
- Alcohols
- Propionates
Wikimedia Foundation. 2010.
Look at other dictionaries:
Atropine — Général Nom IUPAC … Wikipédia en Français
ATROPINE — Substance cristalline, toxique, appartenant à la classe des alcaloïdes. On l’obtient à partir de la L hyociamine qui est un composant de plantes de la famille des Solanaceae telles que la belladone (Atropa ), la jusquiame et le datura. Elle est… … Encyclopédie Universelle
Atropine — At ro*pine, n. [Gr. ? inflexible; hence ? ?, one of the three Parc[ae]; a priv. + ? to turn.] (Chem.) A poisonous, white, crystallizable alkaloid, extracted from the {Atropa belladonna}, or deadly nightshade, and the {Datura Stramonium}, or thorn … The Collaborative International Dictionary of English
atropine — 1836, from L. atropa deadly nightshade (from which the alkaloid poison is extracted), from Gk. atropos inflexible, also the name of one of the Fates (see ATROPOS (Cf. Atropos)) + chemical suffix INE (Cf. ine) (2) … Etymology dictionary
atropine — [at′rəpin΄at′rə pēn΄, at′rəpin΄] n. [< ModL Atropa, genus name of belladonna < Gr Atropos (see ATROPOS) + INE3] a poisonous, crystalline alkaloid, C17H23NO3, obtained from belladonna and similar plants: used to relieve spasms and dilate the … English World dictionary
atropine — /a treuh peen , pin/, n. Pharm. a poisonous crystalline alkaloid, C17H23NO3, obtained from belladonna and other plants of the nightshade family, that prevents the response of various body structures to certain types of nerve stimulation: used… … Universalium
Atropine — A drug obtained from belladonna that is administered via injection, eye drops, or in oral form to relax muscles by inhibiting nerve responses. Used to dilate the pupils and as an antispasmodic. From the Greek goddess Atropos, the oldest and… … Medical dictionary
atropine — n. an antimuscarinic drug that occurs in deadly nightshade (see belladonna). Because it dilates the pupil and paralyses the ciliary muscle (see cycloplegia, mydriatic), atropine is used in eye examinations and the treatment of anterior uveitis.… … The new mediacal dictionary
atropine — atropinas statusas T sritis chemija apibrėžtis Alkaloidas. formulė C₁₇H₂₃NO₃ atitikmenys: angl. atropine rus. атропин … Chemijos terminų aiškinamasis žodynas
atropine methonitrate — atropine methylnitrate methylatropine nitrate … Medical dictionary
